Role of the HIV-1 envelope transmembrane domain in intracellular sorting
- PMID: 29544440
- PMCID: PMC5856207
- DOI: 10.1186/s12860-018-0153-4
Role of the HIV-1 envelope transmembrane domain in intracellular sorting
Abstract
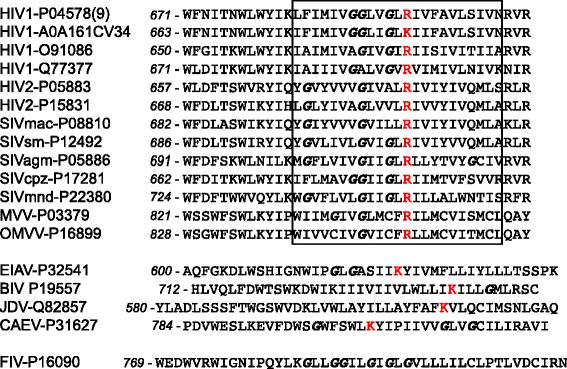
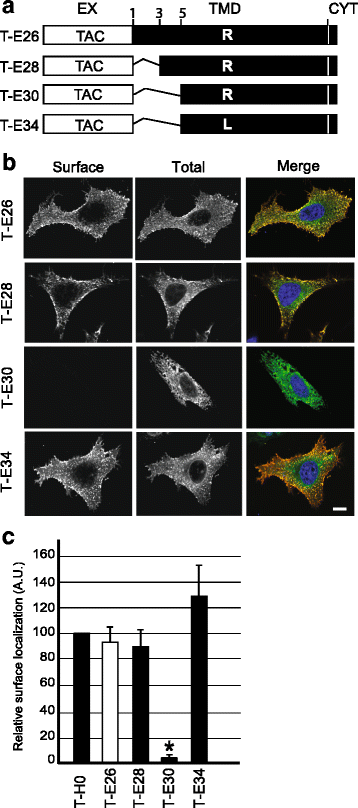
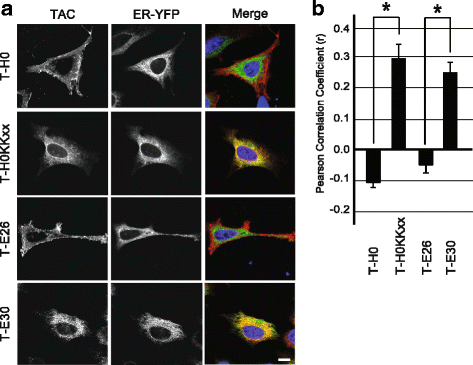
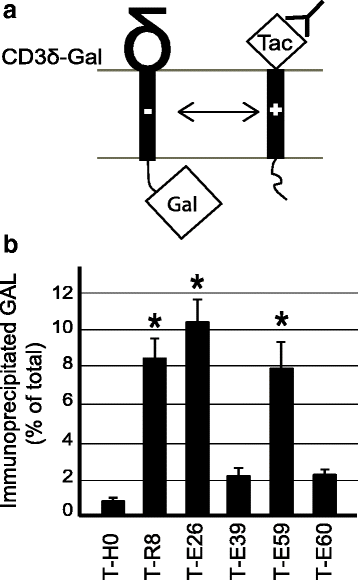
Background: The envelope protein of lentiviruses are type I transmembrane proteins, and their transmembrane domain contains conserved potentially charged residues. This highly unusual feature would be expected to cause endoplasmic reticulum (ER) localization. The aim of this study was to determine by which means the HIV-1 Env protein is transported to the cell surface although its transmembrane domain contains a conserved arginine residue.
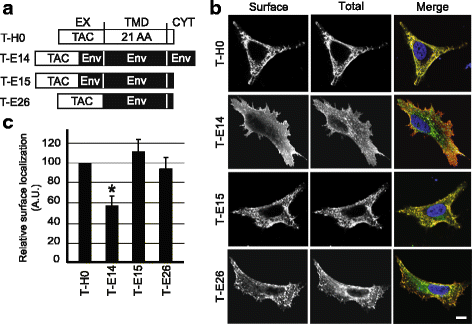
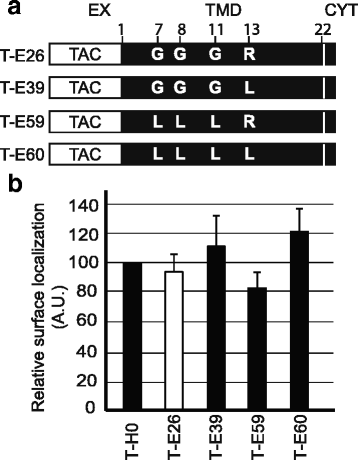
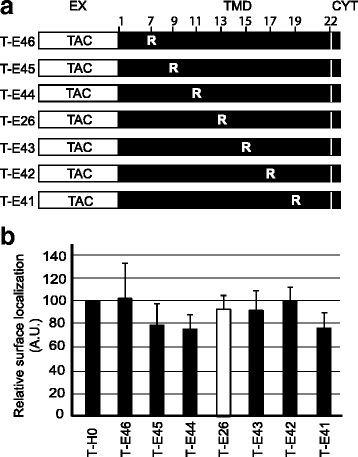
Results: We expressed various chimeric proteins and analyzed the influence of their transmembrane domain on their intracellular localization. The transmembrane domain of the HIV-1 Env protein does not cause ER retention. This is not due to the presence of conserved glycine residues, or to the position of the arginine residue, but to the length of the transmembrane domain. A shortened version of the Env transmembrane domain causes arginine-dependent ER targeting. Remarkably, the transmembrane domain of the HIV-1 Env protein, although it does not confer ER retention, interacts efficiently with negatively charged residues in the membrane.
Conclusion: These results suggest that the intrinsic properties of the HIV-1 Env transmembrane domain allow the protein to escape ER-retention mechanisms, while maintaining its ability to interact with cellular proteins and to influence cellular physiology.
Keywords: Endoplasmic reticulum; Envelope protein; HIV-1; Secretory pathway; Transmembrane domain; gp160.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Analysis of endoproteolytic cleavage and intracellular transport of human immunodeficiency virus type 1 envelope glycoproteins using mutant CD4 molecules bearing the transmembrane endoplasmic reticulum retention signal.J Gen Virol. 1993 Oct;74 ( Pt 10):2085-97. doi: 10.1099/0022-1317-74-10-2085. J Gen Virol. 1993. PMID: 8409933
-
ERManI (Endoplasmic Reticulum Class I α-Mannosidase) Is Required for HIV-1 Envelope Glycoprotein Degradation via Endoplasmic Reticulum-associated Protein Degradation Pathway.J Biol Chem. 2015 Sep 4;290(36):22184-92. doi: 10.1074/jbc.M115.675207. Epub 2015 Jul 23. J Biol Chem. 2015. PMID: 26205822 Free PMC article.
-
HIV-1 Envelope Glycoprotein Trafficking through the Endosomal Recycling Compartment Is Required for Particle Incorporation.J Virol. 2018 Feb 12;92(5):e01893-17. doi: 10.1128/JVI.01893-17. Print 2018 Mar 1. J Virol. 2018. PMID: 29212940 Free PMC article.
-
The human immunodeficiency virus type 1 Vpu protein tethered to the CD4 extracellular domain is localized to the plasma membrane and is biologically active in the secretory pathway of mammalian cells: implications for the mechanisms of Vpu function.Virology. 1996 Jun 1;220(1):141-51. doi: 10.1006/viro.1996.0294. Virology. 1996. PMID: 8659106
-
Retention of the human immunodeficiency virus type 1 envelope glycoprotein in the endoplasmic reticulum does not redirect virus assembly from the plasma membrane.J Virol. 1998 Sep;72(9):7523-31. doi: 10.1128/JVI.72.9.7523-7531.1998. J Virol. 1998. PMID: 9696849 Free PMC article.
Cited by
-
Identification of the endoplasmic reticulum localization sequence and N-glycosylation of matrix metalloproteinase 26.RSC Adv. 2019 Jul 25;9(40):23053-23060. doi: 10.1039/c9ra05222d. eCollection 2019 Jul 23. RSC Adv. 2019. PMID: 35514513 Free PMC article.
-
Viral Membrane Fusion and the Transmembrane Domain.Viruses. 2020 Jun 27;12(7):693. doi: 10.3390/v12070693. Viruses. 2020. PMID: 32604992 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

