Dose escalation can maximize therapeutic potential of sunitinib in patients with metastatic renal cell carcinoma
- PMID: 29544452
- PMCID: PMC5856318
- DOI: 10.1186/s12885-018-4209-9
Dose escalation can maximize therapeutic potential of sunitinib in patients with metastatic renal cell carcinoma
Abstract
Background: In patients with metastatic renal cell cancer, based on limited evidence, increased sunitinib exposure is associated with better outcome. The survival and toxicity data of patients receiving individualized dose escalated sunitinib therapy as compared to standard management were analyzed in this study.
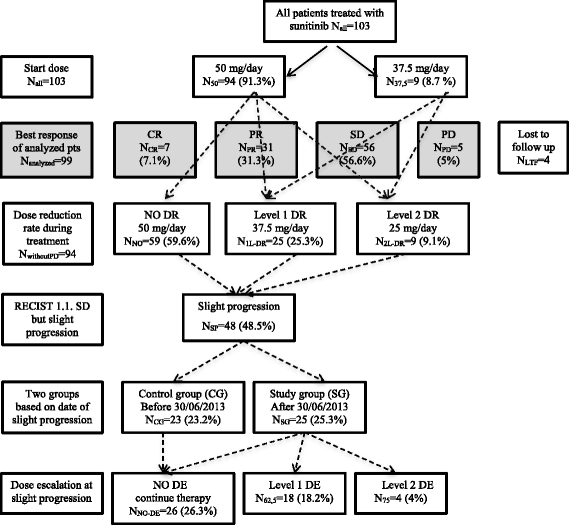
Methods: From July 2013, the data of metastatic renal cell cancer patients with slight progression but still a stable disease according to RECIST 1.1 criteria treated with an escalated dose of sunitinib (first level: 62.5 mg/day in 4/2 or 2 × 2/1 scheme, second level: 75 mg/day in 4/2 or 2 × 2/1 scheme) were collected prospectively. Regarding characteristics, outcome, and toxicity data, an explorative retrospective analysis of the register was carried out, comparing treatments after and before July 1, 2013 in the study (selected patients for escalated dose) and control (standard dose) groups, respectively.
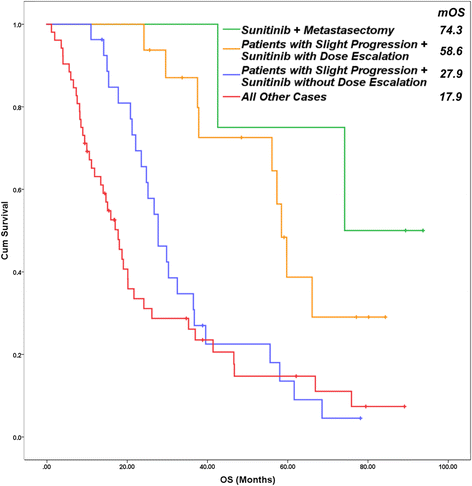
Results: The study involved 103 patients receiving sunitinib therapy with a median overall and progression free survival of 25.36 ± 2.62 and 14.2 ± 3.22 months, respectively. Slight progression was detected in 48.5% of them. First and second-level dose escalation were indicated in 18.2% and 4.1% of patients, respectively. The dosing scheme was modified in 22.2%. The median progression free survival (39.7 ± 5.1 vs 14.2 ± 1.3 months (p = 0.037)) and the overall survival (57.5 ± 10.7 vs 27.9 ± 2.5 months (p = 0.044)) were significantly better in the study group (with dose escalation) than in the control group. Patients with nephrectomy and lower Memorial Sloan Kettering Cancer Center (MSKCC) scores showed more favorable outcomes. After dose escalation, the most common adverse events were worsening or development of fatigue, hypertension, stomatitis, and weight loss of over 10%.
Conclusions: Escalation of sunitinib dosing in selected patients with metastatic renal cell cancer, especially in case of slight progression, based on tolerable toxicity is safe and improves outcome. Dose escalation in 12.5 mg steps may be recommended for properly educated patients.
Keywords: Dose escalation; Improved outcome; Metastatic renal cell cancer; Sunitinib; Toxicity.
Conflict of interest statement
Ethics approval and consent to participate
All the procedures performed were in full accordance with the ethical standards of the appropriate national and institutional committees on human experimentation and with the Helsinki Declaration. The study was approved by the Regional Human Biomedical Research Ethics Committee, Albert Szent-Györgyi Health Center, University of Szeged, Hungary (registration number: WHO 3482/2014). The enrolled patients gave their written informed consent before being registered in the study.
Consent for publication
Not applicable.
Competing interests
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Clinical Experience of Escalated Sunitinib Dose in Select Patients With Metastatic Renal Cell Carcinoma.Clin Genitourin Cancer. 2017 Feb;15(1):139-144. doi: 10.1016/j.clgc.2016.05.007. Epub 2016 May 27. Clin Genitourin Cancer. 2017. PMID: 27338518
-
Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial.Lancet Oncol. 2016 Mar;17(3):378-388. doi: 10.1016/S1470-2045(15)00515-X. Epub 2016 Jan 13. Lancet Oncol. 2016. PMID: 26794930 Free PMC article. Clinical Trial.
-
The outcome of patients treated with sunitinib prior to planned nephrectomy in metastatic clear cell renal cancer.Eur Urol. 2011 Sep;60(3):448-54. doi: 10.1016/j.eururo.2011.05.028. Epub 2011 May 17. Eur Urol. 2011. PMID: 21612860
-
Sunitinib rechallenge with dose escalation in progressive metastatic renal cell carcinoma: A case report and literature review.Medicine (Baltimore). 2018 Aug;97(31):e11565. doi: 10.1097/MD.0000000000011565. Medicine (Baltimore). 2018. PMID: 30075524 Free PMC article. Review.
-
How clinical practice is changing the rules: the sunitinib 2/1 schedule in metastatic renal cell carcinoma.Expert Rev Anticancer Ther. 2017 Mar;17(3):227-233. doi: 10.1080/14737140.2017.1276830. Epub 2017 Jan 3. Expert Rev Anticancer Ther. 2017. PMID: 28044472 Review.
Cited by
-
Systematic literature review of real-world evidence on overall survival in cancer patients before and after the approval of anti-PD-(L)1 therapy.Front Oncol. 2025 Aug 4;15:1615795. doi: 10.3389/fonc.2025.1615795. eCollection 2025. Front Oncol. 2025. PMID: 40831930 Free PMC article.
-
Assessing the effectiveness and safety of surufatinib versus everolimus or sunitinib in advanced neuroendocrine neoplasms: insights from a real-world, retrospective cohort study using propensity score and inverse probability treatment weighting analysis.J Gastrointest Oncol. 2024 Apr 30;15(2):689-709. doi: 10.21037/jgo-24-218. Epub 2024 Apr 29. J Gastrointest Oncol. 2024. PMID: 38756630 Free PMC article.
References
-
- Sun L, Liang C, Shirazian S, Zhou Y, Miller T, Cui J, et al. Discovery of 5-[5-fluoro-2-oxo-1,2- dihydroindol-(3Z)-ylidenemethyl]-2,4- dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide, a novel tyrosine kinase inhibitor targeting vascular endothelial and platelet-derived growth factor receptor tyrosine kinase. J Med Chem. 2003;46(7):1116–1119. doi: 10.1021/jm0204183. - DOI - PubMed
-
- Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9(1):327–337. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

