Statistical analysis plan for the Pneumatic CompREssion for PreVENting Venous Thromboembolism (PREVENT) trial: a study protocol for a randomized controlled trial
- PMID: 29544550
- PMCID: PMC5856363
- DOI: 10.1186/s13063-018-2534-6
Statistical analysis plan for the Pneumatic CompREssion for PreVENting Venous Thromboembolism (PREVENT) trial: a study protocol for a randomized controlled trial
Abstract
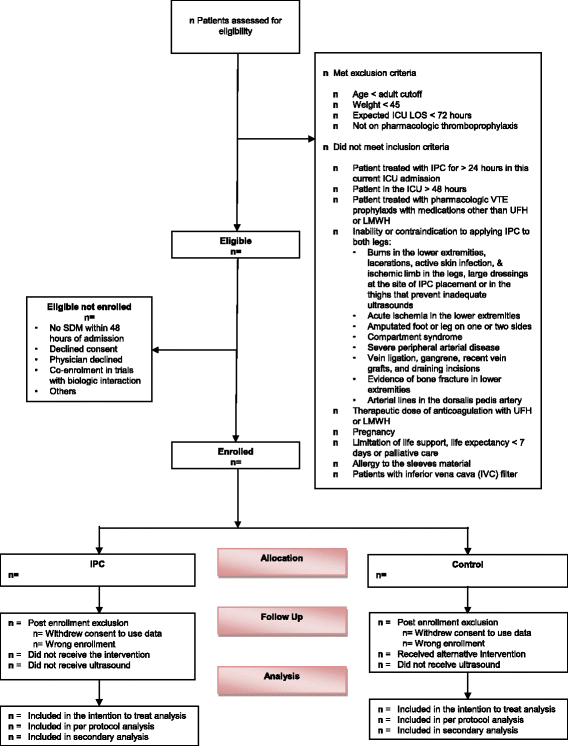
Background: The Pneumatic CompREssion for Preventing VENous Thromboembolism (PREVENT) trial evaluates the effect of adjunctive intermittent pneumatic compression (IPC) with pharmacologic thromboprophylaxis compared to pharmacologic thromboprophylaxis alone on venous thromboembolism (VTE) in critically ill adults.
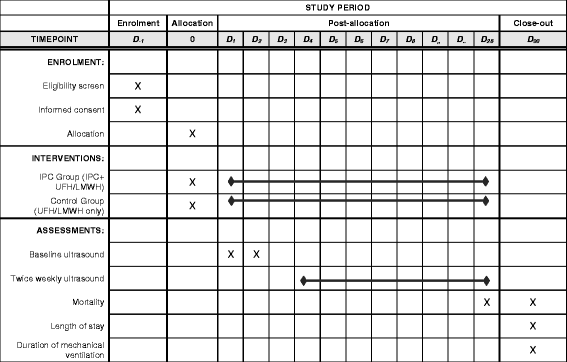
Methods/design: In this multicenter randomized trial, critically ill patients receiving pharmacologic thromboprophylaxis will be randomized to an IPC or a no IPC (control) group. The primary outcome is "incident" proximal lower-extremity deep vein thrombosis (DVT) within 28 days after randomization. Radiologists interpreting the lower-extremity ultrasonography will be blinded to intervention allocation, whereas the patients and treating team will be unblinded. The trial has 80% power to detect a 3% absolute risk reduction in the rate of proximal DVT from 7% to 4%.
Discussion: Consistent with international guidelines, we have developed a detailed plan to guide the analysis of the PREVENT trial. This plan specifies the statistical methods for the evaluation of primary and secondary outcomes, and defines covariates for adjusted analyses a priori. Application of this statistical analysis plan to the PREVENT trial will facilitate unbiased analyses of clinical data.
Trial registration: ClinicalTrials.gov , ID: NCT02040103 . Registered on 3 November 2013; Current controlled trials, ID: ISRCTN44653506 . Registered on 30 October 2013.
Keywords: Adjunct mechanical and pharmacologic DVT prophylaxis; Critically-ill patients; Deep vein thrombosis; Intermittent pneumatic compression; Pulmonary embolism.
Conflict of interest statement
Ethics approval and consent to participate
The study protocol as well as the informed consent have been approved by the Institutional Review Board of King Abdullah International Medical Research Center, King Abdulaziz Medical City, Riyadh and the respective Institutional Review Boards of all the other centers.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e195S–e226S. doi: 10.1378/chest.11-2296. - DOI - PMC - PubMed
-
- Group PIftCCCT, the A, New Zealand Intensive Care Society Clinical Trials G. Cook D, Meade M, Guyatt G, Walter S, Heels-Ansdell D, Warkentin TE, Zytaruk N, et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med. 2011;364(14):1305–14. doi: 10.1056/NEJMoa1014475. - DOI - PubMed
-
- Arabi YM, Khedr M, Dara SI, Dhar GS, Bhat SA, Tamim HM, Afesh LY. Use of intermittent pneumatic compression and not graduated compression stockings is associated with lower incident VTE in critically ill patients: a multiple propensity scores adjusted analysis. Chest. 2013;144(1):152–159. doi: 10.1378/chest.12-2028. - DOI - PubMed
-
- Arabi YM, Alsolamy S, Al-Dawood A, Al-Omari A, Al-Hameed F, Burns KE, Almaani M, Lababidi H, Al Bshabshe A, Mehta S, et al. Thromboprophylaxis using combined intermittent pneumatic compression and pharmacologic prophylaxis versus pharmacologic prophylaxis alone in critically ill patients: study protocol for a randomized controlled trial. Trials. 2016;17(1):390. doi: 10.1186/s13063-016-1520-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical