Impact of pentavalent rotavirus vaccine against severe rotavirus diarrhoea in The Gambia
- PMID: 29544688
- PMCID: PMC6816024
- DOI: 10.1016/j.vaccine.2018.02.091
Impact of pentavalent rotavirus vaccine against severe rotavirus diarrhoea in The Gambia
Abstract
Introduction: Rotavirus vaccines protect against the leading cause of severe childhood diarrhoea, and have been introduced in many low-income African countries. The Gambia introducedRotateq® (RV5) into their national immunization program in 2013. We revieweddata from an active rotavirus sentinel surveillancesitefor early evidence of vaccine impact.
Methods: We compared rotavirus prevalence in diarrhoeal stool in children< 5 years of age admittedat the Edward Francis Small Teaching Hospital sentinel surveillance site before (2013) andafterRV5 introduction (2015-2016) in the Gambia. The rotavirus-percent positive was separately compared for all diarrhoealhospitalizations and for hospitalizations with severe symptoms. Rotavirus prevalence was compared annually for the pre-vaccine year of 2013 with post-vaccine years of 2015 and 2016 using chi-square or Fisher's exact tests and the p-value to establish significant relationship was set at p < 0.05. All analyses were completed in SAS 9.3 (SAS Analytics, North Carolina).
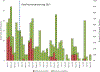
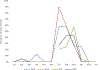
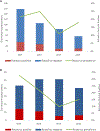
Results: Rotavirus prevalence among all diarrhoeahospitalizations decreased from 22% in 2013 to 11% in 2015 (p = 0.04), while remaining unchanged in 2016 (18%, p = 0.56). For hospitalizations that were clinically severe and/or treated with intravenous fluids (mean of 46 per year), the rotavirus prevalence decreased from 33% in 2013 to 8% in 2015 (p = 0.04), and to 15% in 2016 (p = 0.08). The children with age <1 year accounted for 45% the population infected with rotavirus in both pre and post rotavirus vaccination periods.
Conclusions: Rotavirus vaccine introduction in the Gambia could be among factors resulting in decreased diarrhea hospitalizations among children at the Edward Francis Small Teaching Hospital, particularly those with severe disease. These results support the continuation of rotavirus vaccine and additional monitoring of rotavirus hospitalization trends in the country.
Keywords: Hospitalization; Rotavirus; Severe diarrhoea; Vaccine impact.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of conflict of interest
Authors have no conflict of interest.
Figures
Similar articles
-
Impact of rotavirus vaccine on all-cause diarrhea and rotavirus hospitalizations in Madagascar.Vaccine. 2018 Nov 12;36(47):7198-7204. doi: 10.1016/j.vaccine.2017.08.091. Epub 2017 Sep 25. Vaccine. 2018. PMID: 28958809 Free PMC article.
-
Impact and effectiveness of pentavalent rotavirus vaccine in children <5 years of age in Burkina Faso.Vaccine. 2018 Nov 12;36(47):7170-7178. doi: 10.1016/j.vaccine.2017.12.056. Epub 2017 Dec 28. Vaccine. 2018. PMID: 29290478 Free PMC article.
-
Evidence of the impact of monovalent rotavirus vaccine on childhood acute gastroenteritis hospitalization in Togo.Vaccine. 2018 Nov 12;36(47):7185-7191. doi: 10.1016/j.vaccine.2018.01.058. Epub 2018 Feb 1. Vaccine. 2018. PMID: 29397224 Free PMC article.
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2019 Oct 28;2019(10):CD008521. doi: 10.1002/14651858.CD008521.pub5. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2021 Nov 17;11:CD008521. doi: 10.1002/14651858.CD008521.pub6. PMID: 31684685 Free PMC article. Updated.
-
Vaccines for preventing rotavirus diarrhoea: vaccines in use.Cochrane Database Syst Rev. 2019 Mar 25;3(3):CD008521. doi: 10.1002/14651858.CD008521.pub4. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2019 Oct 28;2019(10). doi: 10.1002/14651858.CD008521.pub5. PMID: 30912133 Free PMC article. Updated.
Cited by
-
Epidemiology and pre-vaccine burden of rotavirus diarrhea in Democratic Republic of Congo (DRC): Results of sentinel surveillance, 2009-2019.Vaccine. 2022 Sep 29;40(41):5933-5941. doi: 10.1016/j.vaccine.2022.08.041. Epub 2022 Sep 6. Vaccine. 2022. PMID: 36068112 Free PMC article.
-
Impact of rotavirus vaccines in Sub-Saharan African countries.Vaccine. 2018 Nov 12;36(47):7119-7123. doi: 10.1016/j.vaccine.2018.06.026. Epub 2018 Jun 18. Vaccine. 2018. PMID: 29914848 Free PMC article.
References
-
- Rotavirus vaccines:an update. Releve epidemiologique hebdomadaire. 2009;84 (50):533–40. - PubMed
-
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England J Med 2010;362(4):289–98. - PubMed
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, doubleblind, placebo-controlled trial. Lancet (London, England). 2010;376 (9741):606–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

