Impact of pentavalent rotavirus vaccine against severe rotavirus diarrhoea in The Gambia
- PMID: 29544688
- PMCID: PMC6816024
- DOI: 10.1016/j.vaccine.2018.02.091
Impact of pentavalent rotavirus vaccine against severe rotavirus diarrhoea in The Gambia
Abstract
Introduction: Rotavirus vaccines protect against the leading cause of severe childhood diarrhoea, and have been introduced in many low-income African countries. The Gambia introducedRotateq® (RV5) into their national immunization program in 2013. We revieweddata from an active rotavirus sentinel surveillancesitefor early evidence of vaccine impact.
Methods: We compared rotavirus prevalence in diarrhoeal stool in children< 5 years of age admittedat the Edward Francis Small Teaching Hospital sentinel surveillance site before (2013) andafterRV5 introduction (2015-2016) in the Gambia. The rotavirus-percent positive was separately compared for all diarrhoealhospitalizations and for hospitalizations with severe symptoms. Rotavirus prevalence was compared annually for the pre-vaccine year of 2013 with post-vaccine years of 2015 and 2016 using chi-square or Fisher's exact tests and the p-value to establish significant relationship was set at p < 0.05. All analyses were completed in SAS 9.3 (SAS Analytics, North Carolina).
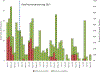
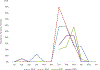
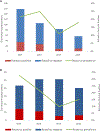
Results: Rotavirus prevalence among all diarrhoeahospitalizations decreased from 22% in 2013 to 11% in 2015 (p = 0.04), while remaining unchanged in 2016 (18%, p = 0.56). For hospitalizations that were clinically severe and/or treated with intravenous fluids (mean of 46 per year), the rotavirus prevalence decreased from 33% in 2013 to 8% in 2015 (p = 0.04), and to 15% in 2016 (p = 0.08). The children with age <1 year accounted for 45% the population infected with rotavirus in both pre and post rotavirus vaccination periods.
Conclusions: Rotavirus vaccine introduction in the Gambia could be among factors resulting in decreased diarrhea hospitalizations among children at the Edward Francis Small Teaching Hospital, particularly those with severe disease. These results support the continuation of rotavirus vaccine and additional monitoring of rotavirus hospitalization trends in the country.
Keywords: Hospitalization; Rotavirus; Severe diarrhoea; Vaccine impact.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of conflict of interest
Authors have no conflict of interest.
Figures
References
-
- Rotavirus vaccines:an update. Releve epidemiologique hebdomadaire. 2009;84 (50):533–40. - PubMed
-
- Madhi SA, Cunliffe NA, Steele D, Witte D, Kirsten M, Louw C, et al. Effect of human rotavirus vaccine on severe diarrhea in African infants. New England J Med 2010;362(4):289–98. - PubMed
-
- Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, doubleblind, placebo-controlled trial. Lancet (London, England). 2010;376 (9741):606–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

