Homing in: Mechanisms of Substrate Targeting by Protein Kinases
- PMID: 29544874
- PMCID: PMC5923429
- DOI: 10.1016/j.tibs.2018.02.009
Homing in: Mechanisms of Substrate Targeting by Protein Kinases
Abstract
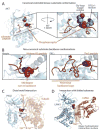
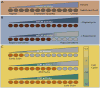
Protein phosphorylation is the most common reversible post-translational modification in eukaryotes. Humans have over 500 protein kinases, of which more than a dozen are established targets for anticancer drugs. All kinases share a structurally similar catalytic domain, yet each one is uniquely positioned within signaling networks controlling essentially all aspects of cell behavior. Kinases are distinguished from one another based on their modes of regulation and their substrate repertoires. Coupling specific inputs to the proper signaling outputs requires that kinases phosphorylate a limited number of sites to the exclusion of hundreds of thousands of off-target phosphorylation sites. Here, we review recent progress in understanding mechanisms of kinase substrate specificity and how they function to shape cellular signaling networks.
Keywords: enzyme specificity; linear sequence motif; protein interactions; protein kinase; protein phosphorylation.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Classification of Protein Kinases Influenced by Conservation of Substrate Binding Residues.Methods Mol Biol. 2016;1415:301-13. doi: 10.1007/978-1-4939-3572-7_15. Methods Mol Biol. 2016. PMID: 27115639
-
Hierarchical Organization Endows the Kinase Domain with Regulatory Plasticity.Cell Syst. 2018 Oct 24;7(4):371-383.e4. doi: 10.1016/j.cels.2018.08.008. Epub 2018 Sep 19. Cell Syst. 2018. PMID: 30243563 Free PMC article.
-
Phosphorylation by LAMMER protein kinases: determination of a consensus site, identification of in vitro substrates, and implications for substrate preferences.Biochemistry. 2002 Feb 12;41(6):2055-66. doi: 10.1021/bi011521h. Biochemistry. 2002. PMID: 11827553
-
Protein kinase structure and function analysis with chemical tools.Biochim Biophys Acta. 2005 Dec 30;1754(1-2):65-78. doi: 10.1016/j.bbapap.2005.08.020. Epub 2005 Sep 13. Biochim Biophys Acta. 2005. PMID: 16213197 Review.
-
Structural Basis for the Non-catalytic Functions of Protein Kinases.Structure. 2016 Jan 5;24(1):7-24. doi: 10.1016/j.str.2015.10.020. Structure. 2016. PMID: 26745528 Free PMC article. Review.
Cited by
-
Proline-Rich Motifs Control G2-CDK Target Phosphorylation and Priming an Anchoring Protein for Polo Kinase Localization.Cell Rep. 2020 Jun 16;31(11):107757. doi: 10.1016/j.celrep.2020.107757. Cell Rep. 2020. PMID: 32553169 Free PMC article.
-
Condensed-phase signaling can expand kinase specificity and respond to macromolecular crowding.Mol Cell. 2022 Oct 6;82(19):3693-3711.e10. doi: 10.1016/j.molcel.2022.08.016. Epub 2022 Sep 14. Mol Cell. 2022. PMID: 36108633 Free PMC article.
-
Saturation mutagenesis of a predicted ancestral Syk-family kinase.Protein Sci. 2022 Oct;31(10):e4411. doi: 10.1002/pro.4411. Protein Sci. 2022. PMID: 36173161 Free PMC article.
-
A Tale of Two Tyrosines.Biophys J. 2020 Nov 17;119(10):1927-1928. doi: 10.1016/j.bpj.2020.09.036. Epub 2020 Oct 14. Biophys J. 2020. PMID: 33120016 Free PMC article. No abstract available.
-
Identification of phosphatases that dephosphorylate the co-chaperone BAG3.Life Sci Alliance. 2024 Nov 19;8(2):e202402734. doi: 10.26508/lsa.202402734. Print 2025 Feb. Life Sci Alliance. 2024. PMID: 39562141 Free PMC article.
References
-
- Ubersax JA, Ferrell JE., Jr Mechanisms of specificity in protein phosphorylation. Nat Rev Mol Cell Biol. 2007;8:530–541. - PubMed
-
- Manning G, et al. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. - PubMed
-
- Bose R, et al. Protein tyrosine kinase–substrate interactions. Curr Opin Struct Biol. 2006;16:668–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

