ICEC0942, an Orally Bioavailable Selective Inhibitor of CDK7 for Cancer Treatment
- PMID: 29545334
- PMCID: PMC5985928
- DOI: 10.1158/1535-7163.MCT-16-0847
ICEC0942, an Orally Bioavailable Selective Inhibitor of CDK7 for Cancer Treatment
Abstract
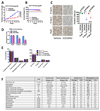
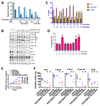
Recent reports indicate that some cancer types are especially sensitive to transcription inhibition, suggesting that targeting the transcriptional machinery provides new approaches to cancer treatment. Cyclin-dependent kinase (CDK)7 is necessary for transcription, and acts by phosphorylating the C-terminal domain (CTD) of RNA polymerase II (PolII) to enable transcription initiation. CDK7 additionally regulates the activities of a number of transcription factors, including estrogen receptor (ER)-α. Here we describe a new, orally bioavailable CDK7 inhibitor, ICEC0942. It selectively inhibits CDK7, with an IC50 of 40 nmol/L; IC50 values for CDK1, CDK2, CDK5, and CDK9 were 45-, 15-, 230-, and 30-fold higher. In vitro studies show that a wide range of cancer types are sensitive to CDK7 inhibition with GI50 values ranging between 0.2 and 0.3 μmol/L. In xenografts of both breast and colorectal cancers, the drug has substantial antitumor effects. In addition, combination therapy with tamoxifen showed complete growth arrest of ER-positive tumor xenografts. Our findings reveal that CDK7 inhibition provides a new approach, especially for ER-positive breast cancer and identify ICEC0942 as a prototype drug with potential utility as a single agent or in combination with hormone therapies for breast cancer. ICEC0942 may also be effective in other cancers that display characteristics of transcription factor addiction, such as acute leukaemia and small-cell lung cancer. Mol Cancer Ther; 17(6); 1156-66. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures
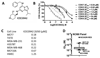


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

