Cytokines increase engraftment of human acute myeloid leukemia cells in immunocompromised mice but not engraftment of human myelodysplastic syndrome cells
- PMID: 29545344
- PMCID: PMC6058784
- DOI: 10.3324/haematol.2017.183202
Cytokines increase engraftment of human acute myeloid leukemia cells in immunocompromised mice but not engraftment of human myelodysplastic syndrome cells
Abstract
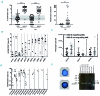
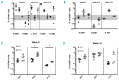
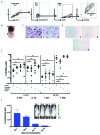
Patient-derived xenotransplantation models of human myeloid diseases including acute myeloid leukemia, myelodysplastic syndromes and myeloproliferative neoplasms are essential for studying the biology of the diseases in pre-clinical studies. However, few studies have used these models for comparative purposes. Previous work has shown that acute myeloid leukemia blasts respond to human hematopoietic cytokines whereas myelodysplastic syndrome cells do not. We compared the engraftment of acute myeloid leukemia cells and myelodysplastic syndrome cells in NSG mice to that in NSG-S mice, which have transgene expression of human cytokines. We observed that only 50% of all primary acute myeloid leukemia samples (n=77) transplanted in NSG mice provided useful levels of engraftment (>0.5% human blasts in bone marrow). In contrast, 82% of primary acute myeloid leukemia samples engrafted in NSG-S mice with higher leukemic burden and shortened survival. Additionally, all of 5 injected samples from patients with myelodysplastic syndrome showed persistent engraftment on week 6; however, engraftment was mostly low (<2%), did not increase over time, and was only transiently affected by the use of NSG-S mice. Co-injection of mesenchymal stem cells did not enhance human myelodysplastic syndrome cell engraftment. Overall, we conclude that engraftment of acute myeloid leukemia samples is more robust compared to that of myelodysplastic syndrome samples and unlike those, acute myeloid leukemia cells respond positively to human cytokines, whereas myelodysplastic syndrome cells demonstrate a general unresponsiveness to them.
Copyright © 2018 Ferrata Storti Foundation.
Figures



Comment in
-
NSG-S mice for acute myeloid leukemia, yes. For myelodysplastic syndrome, no.Haematologica. 2018 Jun;103(6):921-923. doi: 10.3324/haematol.2018.193847. Haematologica. 2018. PMID: 29866886 Free PMC article. No abstract available.
References
-
- Tallman MS, Gilliland DG, Rowe JM. Drug therapy for acute myeloid leukemia. Blood. 2005;106(4):1154–1163. - PubMed
-
- Woll PS, Kjällquist U, Chowdhury O, et al. Myelodysplastic syndromes are propagated by rare and distinct human cancer stem cells in vivo. Cancer Cell. 2014;25(6):794–808. - PubMed
-
- Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4(1–2):7–25. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

