Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry
- PMID: 29545366
- PMCID: PMC5993603
- DOI: 10.1161/CIRCRESAHA.117.312513
Atlas of the Immune Cell Repertoire in Mouse Atherosclerosis Defined by Single-Cell RNA-Sequencing and Mass Cytometry
Abstract
Rationale: Atherosclerosis is a chronic inflammatory disease that is driven by the interplay of pro- and anti-inflammatory leukocytes in the aorta. Yet, the phenotypic and transcriptional diversity of aortic leukocytes is poorly understood.
Objective: We characterized leukocytes from healthy and atherosclerotic mouse aortas in-depth by single-cell RNA-sequencing and mass cytometry (cytometry by time of flight) to define an atlas of the immune cell landscape in atherosclerosis.
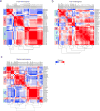
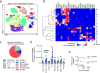
Methods and results: Using single-cell RNA-sequencing of aortic leukocytes from chow diet- and Western diet-fed Apoe-/- and Ldlr-/- mice, we detected 11 principal leukocyte clusters with distinct phenotypic and spatial characteristics while the cellular repertoire in healthy aortas was less diverse. Gene set enrichment analysis on the single-cell level established that multiple pathways, such as for lipid metabolism, proliferation, and cytokine secretion, were confined to particular leukocyte clusters. Leukocyte populations were differentially regulated in atherosclerotic Apoe-/- and Ldlr-/- mice. We confirmed the phenotypic diversity of these clusters with a novel mass cytometry 35-marker panel with metal-labeled antibodies and conventional flow cytometry. Cell populations retrieved by these protein-based approaches were highly correlated to transcriptionally defined clusters. In an integrated screening strategy of single-cell RNA-sequencing, mass cytometry, and fluorescence-activated cell sorting, we detected 3 principal B-cell subsets with alterations in surface markers, functional pathways, and in vitro cytokine secretion. Leukocyte cluster gene signatures revealed leukocyte frequencies in 126 human plaques by a genetic deconvolution strategy. This approach revealed that human carotid plaques and microdissected mouse plaques were mostly populated by macrophages, T-cells, and monocytes. In addition, the frequency of genetically defined leukocyte populations in carotid plaques predicted cardiovascular events in patients.
Conclusions: The definition of leukocyte diversity by high-dimensional analyses enables a fine-grained analysis of aortic leukocyte subsets, reveals new immunologic mechanisms and cell-type-specific pathways, and establishes a functional relevance for lesional leukocytes in human atherosclerosis.
Keywords: atherosclerosis; flow cytometry; immune system; leukocytes; lymphocytes; macrophages; mass cytometry; single-cell RNA-sequencing.
© 2018 American Heart Association, Inc.
Conflict of interest statement
All authors have declared that no conflict of interest exists.
Figures





Comment in
-
Redefining leukocytes in atherosclerosis.Nat Rev Cardiol. 2018 Jun;15(6):319. doi: 10.1038/s41569-018-0010-3. Nat Rev Cardiol. 2018. PMID: 29636546 No abstract available.
-
First Giant Steps Toward a Cell Atlas of Atherosclerosis.Circ Res. 2018 Jun 8;122(12):1632-1634. doi: 10.1161/CIRCRESAHA.118.313076. Circ Res. 2018. PMID: 29880495 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

