Inhibition of Glycolysis in Prostate Cancer Chemoprevention by Phenethyl Isothiocyanate
- PMID: 29545400
- PMCID: PMC5984685
- DOI: 10.1158/1940-6207.CAPR-17-0389
Inhibition of Glycolysis in Prostate Cancer Chemoprevention by Phenethyl Isothiocyanate
Abstract
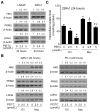
We have shown previously that dietary administration of phenethyl isothiocyanate (PEITC), a small molecule from edible cruciferous vegetables, significantly decreases the incidence of poorly differentiated prostate cancer in Transgenic Adenocarcinoma of Mouse Prostate (TRAMP) mice without any side effects. In this study, we investigated the role of c-Myc-regulated glycolysis in prostate cancer chemoprevention by PEITC. Exposure of LNCaP (androgen-responsive) and 22Rv1 (castration-resistant) human prostate cancer cells to PEITC resulted in suppression of expression as well as transcriptional activity of c-Myc. Prostate cancer cell growth inhibition by PEITC was significantly attenuated by stable overexpression of c-Myc. Analysis of the RNA-Seq data from The Cancer Genome Atlas indicated a significant positive association between Myc expression and gene expression of many glycolysis-related genes, including hexokinase II and lactate dehydrogenase A Expression of these enzyme proteins and lactate levels were decreased upon PEITC treatment in prostate cancer cells, and these effects were significantly attenuated by ectopic expression of c-Myc. A normal prostate stromal cell line (PrSC) was resistant to lactic acid suppression by PEITC treatment. Prostate cancer chemoprevention by PEITC in TRAMP mice was associated with a significant decrease in plasma lactate and pyruvate levels. However, a 1-week intervention with 10 mg PEITC (orally, 4 times/day) was not sufficient to decrease lactate levels in the serum of human subjects. These results indicated that although prostate cancer prevention by PEITC in TRAMP mice was associated with suppression of glycolysis, longer than 1-week intervention might be necessary to observe such an effect in human subjects. Cancer Prev Res; 11(6); 337-46. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures





Similar articles
-
Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane.Carcinogenesis. 2019 Dec 31;40(12):1545-1556. doi: 10.1093/carcin/bgz155. Carcinogenesis. 2019. PMID: 31555797 Free PMC article.
-
Fatty Acid Synthesis Intermediates Represent Novel Noninvasive Biomarkers of Prostate Cancer Chemoprevention by Phenethyl Isothiocyanate.Cancer Prev Res (Phila). 2017 May;10(5):279-289. doi: 10.1158/1940-6207.CAPR-17-0001. Epub 2017 Mar 14. Cancer Prev Res (Phila). 2017. PMID: 28292742 Free PMC article.
-
Chemopreventative potential of the cruciferous vegetable constituent phenethyl isothiocyanate in a mouse model of prostate cancer.J Natl Cancer Inst. 2011 Apr 6;103(7):571-84. doi: 10.1093/jnci/djr029. Epub 2011 Feb 17. J Natl Cancer Inst. 2011. PMID: 21330634 Free PMC article.
-
Prostate cancer chemopreventive activity of phenethyl isothiocyanate through epigenetic regulation (review).Int J Oncol. 2010 Sep;37(3):533-9. doi: 10.3892/ijo_00000702. Int J Oncol. 2010. PMID: 20664922 Review.
-
Phenethyl isothiocyanate: a comprehensive review of anti-cancer mechanisms.Biochim Biophys Acta. 2014 Dec;1846(2):405-24. doi: 10.1016/j.bbcan.2014.08.003. Epub 2014 Aug 23. Biochim Biophys Acta. 2014. PMID: 25152445 Free PMC article. Review.
Cited by
-
Lysine Acetyltransferases and Their Role in AR Signaling and Prostate Cancer.Front Endocrinol (Lausanne). 2022 Aug 17;13:886594. doi: 10.3389/fendo.2022.886594. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36060957 Free PMC article. Review.
-
Reversal of the Warburg phenomenon in chemoprevention of prostate cancer by sulforaphane.Carcinogenesis. 2019 Dec 31;40(12):1545-1556. doi: 10.1093/carcin/bgz155. Carcinogenesis. 2019. PMID: 31555797 Free PMC article.
-
The Anti-AGEing and RAGEing Potential of Isothiocyanates.Molecules. 2024 Dec 19;29(24):5986. doi: 10.3390/molecules29245986. Molecules. 2024. PMID: 39770075 Free PMC article. Review.
-
Natural products targeting glycolysis in cancer.Front Pharmacol. 2022 Nov 1;13:1036502. doi: 10.3389/fphar.2022.1036502. eCollection 2022. Front Pharmacol. 2022. PMID: 36386122 Free PMC article.
-
Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers.Molecules. 2018 Nov 15;23(11):2983. doi: 10.3390/molecules23112983. Molecules. 2018. PMID: 30445746 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

