Assessment of intrinsic and extrinsic signaling pathway in excitotoxic retinal ganglion cell death
- PMID: 29545615
- PMCID: PMC5854579
- DOI: 10.1038/s41598-018-22848-y
Assessment of intrinsic and extrinsic signaling pathway in excitotoxic retinal ganglion cell death
Abstract
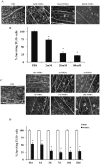
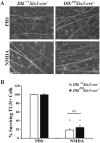
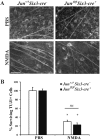
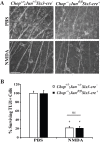
Excitotoxicity leads to the activation of a cytotoxic cascade that causes neuronal death. In the retina, retinal ganglion cells (RGCs) die after an excitotoxic insult. Multiple pathways have been proposed to contribute to RGC death after an excitotoxic insult, including TNF signaling, JNK activation, and ER stress. To test the importance of these pathways in RGC death after excitotoxic injury, the excitotoxin N-methyl-D-aspartate (NMDA) was intravitreally injected into mice deficient in components of these pathways. Absence of Tnf or its canonical downstream mediator, Bid, did not confer short- or long-term protection to RGCs. Despite known activation in RGCs and a prominent role in mediating RGC death after other insults, attenuating JNK signaling did not prevent RGC death after excitotoxic insult. Additionally, deficiency of the ER stress protein DDIT3 (CHOP), which has been shown to be involved in RGC death, did not lessen NMDA induced RGC death. Furthermore, absence of both Jun (JNK's canonical target) and Ddit3, which together provide robust, long-term protection to RGC somas after axonal insult, did not lessen RGC death. Collectively, these results indicate that the drivers of excitotoxic injury remain to be identified and/or multiple cell death pathways are activated in response to injury.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Together JUN and DDIT3 (CHOP) control retinal ganglion cell death after axonal injury.Mol Neurodegener. 2017 Oct 2;12(1):71. doi: 10.1186/s13024-017-0214-8. Mol Neurodegener. 2017. PMID: 28969695 Free PMC article.
-
Endothelin 1-induced retinal ganglion cell death is largely mediated by JUN activation.Cell Death Dis. 2020 Sep 26;11(9):811. doi: 10.1038/s41419-020-02990-0. Cell Death Dis. 2020. PMID: 32980857 Free PMC article.
-
JNK2 and JNK3 are major regulators of axonal injury-induced retinal ganglion cell death.Neurobiol Dis. 2012 May;46(2):393-401. doi: 10.1016/j.nbd.2012.02.003. Epub 2012 Feb 14. Neurobiol Dis. 2012. PMID: 22353563 Free PMC article.
-
Targeting excitotoxic/free radical signaling pathways for therapeutic intervention in glaucoma.Prog Brain Res. 2008;173:495-510. doi: 10.1016/S0079-6123(08)01134-5. Prog Brain Res. 2008. PMID: 18929130 Review.
-
Melatonin As a Modulator of Degenerative and Regenerative Signaling Pathways in Injured Retinal Ganglion Cells.Curr Pharm Des. 2019;25(28):3057-3073. doi: 10.2174/1381612825666190829151314. Curr Pharm Des. 2019. PMID: 31465274 Review.
Cited by
-
Evaluation of the neuroprotective efficacy of the gramine derivative ITH12657 against NMDA-induced excitotoxicity in the rat retina.Front Neuroanat. 2024 Feb 13;18:1335176. doi: 10.3389/fnana.2024.1335176. eCollection 2024. Front Neuroanat. 2024. PMID: 38415017 Free PMC article.
-
Targeting Polyamine Oxidase to Prevent Excitotoxicity-Induced Retinal Neurodegeneration.Front Neurosci. 2019 Jan 10;12:956. doi: 10.3389/fnins.2018.00956. eCollection 2018. Front Neurosci. 2019. PMID: 30686964 Free PMC article.
-
Melanopsin+RGCs Are fully Resistant to NMDA-Induced Excitotoxicity.Int J Mol Sci. 2019 Jun 20;20(12):3012. doi: 10.3390/ijms20123012. Int J Mol Sci. 2019. PMID: 31226772 Free PMC article.
-
Carotenoids in the Management of Glaucoma: A Systematic Review of the Evidence.Nutrients. 2021 Jun 6;13(6):1949. doi: 10.3390/nu13061949. Nutrients. 2021. PMID: 34204051 Free PMC article.
-
Novel roles of ER stress in repressing neural activity and seizures through Mdm2- and p53-dependent protein translation.PLoS Genet. 2019 Sep 26;15(9):e1008364. doi: 10.1371/journal.pgen.1008364. eCollection 2019 Sep. PLoS Genet. 2019. PMID: 31557161 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

