Intravenous C16 and angiopoietin-1 improve the efficacy of placenta-derived mesenchymal stem cell therapy for EAE
- PMID: 29545630
- PMCID: PMC5854598
- DOI: 10.1038/s41598-018-22867-9
Intravenous C16 and angiopoietin-1 improve the efficacy of placenta-derived mesenchymal stem cell therapy for EAE
Erratum in
-
Author Correction: Intravenous C16 and angiopoietin-1 improve the efficacy of placenta-derived mesenchymal stem cell therapy for EAE.Sci Rep. 2020 May 12;10(1):8097. doi: 10.1038/s41598-020-65138-2. Sci Rep. 2020. PMID: 32393781 Free PMC article.
Abstract
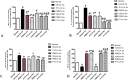
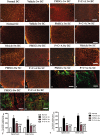
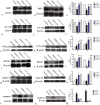
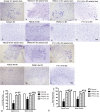
The placenta has emerged as an attractive source of mesenchymal stem cells (MSCs) because of the absence of ethical issues, non-invasive access, and abundant yield. However, inflammatory cell invasion into grafts negatively impacts the survival and efficacy of transplanted cells. Previous studies have shown that synthetic C16 peptide can competitively block the transmigration of leukocytes into the central nerve system, while angiopoietin-1 (Ang-1) can inhibit inflammation-induced blood vessel leakage and inflammatory cell infiltration in rats with experimental allergic encephalomyelitis (EAE). In this study, we investigated the effects of intravenous administration of C16 and Ang-1 on the efficacy of placenta-derived MSC (PMSC) transplantation in a rat model of EAE. We found that, compared with PMSCs alone, treatment with PMSCs along with intravenously administered C16 and Ang-1 was more effective at ameliorating demyelination/neuronal loss and neurological dysfunction, reducing inflammatory cell infiltration, perivascular edema, and reactive astrogliosis (p < 0.05). Mechanistic studies revealed that intravenous C16 and Ang-1 increased PMSC engraftment in the central nervous system and promoted expression of the neurotropic proteins brain-derived neurotrophic factor, growth-associated protein 43, and p75 neurotrophin receptor as well as the neuronal-glial lineage markers neurofilament protein 200 and myelin basic protein in the engrafted PMSCs.
Conflict of interest statement
The authors declare no competing interests.
Figures






Similar articles
-
Amelioration of experimental autoimmune encephalomyelitis through transplantation of placental derived mesenchymal stem cells.Sci Rep. 2017 Feb 10;7:41837. doi: 10.1038/srep41837. Sci Rep. 2017. PMID: 28186117 Free PMC article.
-
Role of C16, angiopoietin-1 and regeneration gene protein 2 in attenuating inflammation in an experimental rat model of autoimmune encephalomyelitis.J Anat. 2017 Jan;230(1):30-46. doi: 10.1111/joa.12541. Epub 2016 Oct 19. J Anat. 2017. PMID: 27757964 Free PMC article.
-
Neuroprotective effects of placenta-derived mesenchymal stromal cells in a rat model of experimental autoimmune encephalomyelitis.Cytotherapy. 2016 Sep;18(9):1100-13. doi: 10.1016/j.jcyt.2016.06.002. Epub 2016 Jul 15. Cytotherapy. 2016. PMID: 27425406
-
Adipose-derived mesenchymal stem cells modulate the immune response in chronic experimental autoimmune encephalomyelitis model.IUBMB Life. 2016 Feb;68(2):106-15. doi: 10.1002/iub.1469. Epub 2016 Jan 12. IUBMB Life. 2016. PMID: 26757144 Review.
-
Mesenchymal stem cells as a treatment for multiple sclerosis: a focus on experimental animal studies.Rev Neurosci. 2020 Jan 28;31(2):161-179. doi: 10.1515/revneuro-2019-0040. Rev Neurosci. 2020. PMID: 31605598 Review.
Cited by
-
The Psoriasis Therapeutic Potential of a Novel Short Laminin Peptide C16.Int J Mol Sci. 2019 Jun 27;20(13):3144. doi: 10.3390/ijms20133144. Int J Mol Sci. 2019. PMID: 31252620 Free PMC article.
-
The therapeutic applications of mesenchymal stromal cells from human perinatal tissues in autoimmune diseases.Stem Cell Res Ther. 2021 Feb 4;12(1):103. doi: 10.1186/s13287-021-02158-3. Stem Cell Res Ther. 2021. PMID: 33541422 Free PMC article. Review.
-
Adjusting vascular permeability, leukocyte infiltration, and microglial cell activation to rescue dopaminergic neurons in rodent models of Parkinson's disease.NPJ Parkinsons Dis. 2021 Oct 8;7(1):91. doi: 10.1038/s41531-021-00233-3. NPJ Parkinsons Dis. 2021. PMID: 34625569 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

