Death Receptor 3 Signaling Controls the Balance between Regulatory and Effector Lymphocytes in SAMP1/YitFc Mice with Crohn's Disease-Like Ileitis
- PMID: 29545797
- PMCID: PMC5837992
- DOI: 10.3389/fimmu.2018.00362
Death Receptor 3 Signaling Controls the Balance between Regulatory and Effector Lymphocytes in SAMP1/YitFc Mice with Crohn's Disease-Like Ileitis
Abstract
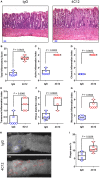
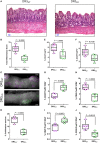
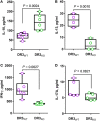
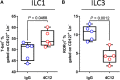
Death receptor 3 (DR3), a member of the tumor necrosis factor receptor (TNFR) superfamily, has been implicated in regulating T-helper type-1 (TH1), type-2 (TH2), and type-17 (TH17) responses as well as regulatory T cell (Treg) and innate lymphoid cell (ILC) functions during immune-mediated diseases. However, the role of DR3 in controlling lymphocyte functions in inflammatory bowel disease (IBD) is not fully understood. Recent studies have shown that activation of DR3 signaling modulates Treg expansion suggesting that stimulation of DR3 represents a potential therapeutic target in human inflammatory diseases, including Crohn's disease (CD). In this study, we tested a specific DR3 agonistic antibody (4C12) in SAMP1/YitFc (SAMP) mice with CD-like ileitis. Interestingly, treatment with 4C12 prior to disease manifestation markedly worsened the severity of ileitis in SAMP mice despite an increase in FoxP3+ lymphocytes in mesenteric lymph node (MLN) and small-intestinal lamina propria (LP) cells. Disease exacerbation was dominated by overproduction of both TH1 and TH2 cytokines and associated with expansion of dysfunctional CD25-FoxP3+ and ILC group 1 (ILC1) cells. These effects were accompanied by a reduction in CD25+FoxP3+ and ILC group 3 (ILC3) cells. By comparison, genetic deletion of DR3 effectively reversed the inflammatory phenotype in SAMP mice by promoting the expansion of CD25+FoxP3+ over CD25-FoxP3+ cells and the production of IL-10 protein. Collectively, our data demonstrate that DR3 signaling modulates a multicellular network, encompassing Tregs, T effectors, and ILCs, governing disease development and progression in SAMP mice with CD-like ileitis. Manipulating DR3 signaling toward the restoration of the balance between protective and inflammatory lymphocytes may represent a novel and targeted therapeutic modality for patients with CD.
Keywords: CD25+/− T cells; Crohn’s disease; SAMP1/YitFc; TL1A; death receptor 3; ileitis; inflammatory bowel disease; innate lymphoid cell; regulatory T cells.
Figures



Similar articles
-
Tregs are dysfunctional in vivo in a spontaneous murine model of Crohn's disease.Mucosal Immunol. 2013 Mar;6(2):267-75. doi: 10.1038/mi.2012.67. Epub 2012 Jul 11. Mucosal Immunol. 2013. PMID: 22785225 Free PMC article.
-
Death-Domain-Receptor 3 Deletion Normalizes Inflammatory Gene Expression and Prevents Ileitis in Experimental Crohn's Disease.Inflamm Bowel Dis. 2019 Jan 1;25(1):14-26. doi: 10.1093/ibd/izy305. Inflamm Bowel Dis. 2019. PMID: 30295722 Free PMC article.
-
TL1A (TNFSF15) and DR3 (TNFRSF25): A Co-stimulatory System of Cytokines With Diverse Functions in Gut Mucosal Immunity.Front Immunol. 2019 Mar 27;10:583. doi: 10.3389/fimmu.2019.00583. eCollection 2019. Front Immunol. 2019. PMID: 30972074 Free PMC article. Review.
-
SAMP1/YitFc mice develop ileitis via loss of CCL21 and defects in dendritic cell migration.Gastroenterology. 2015 Apr;148(4):783-793.e5. doi: 10.1053/j.gastro.2015.01.027. Epub 2015 Jan 22. Gastroenterology. 2015. PMID: 25620669 Free PMC article.
-
Tumor Necrosis Factor-like Cytokine TL1A and Its Receptors DR3 and DcR3: Important New Factors in Mucosal Homeostasis and Inflammation.Inflamm Bowel Dis. 2015 Oct;21(10):2441-52. doi: 10.1097/MIB.0000000000000492. Inflamm Bowel Dis. 2015. PMID: 26099067 Review.
Cited by
-
Role of TL1A in Inflammatory Autoimmune Diseases: A Comprehensive Review.Front Immunol. 2022 Jul 14;13:891328. doi: 10.3389/fimmu.2022.891328. eCollection 2022. Front Immunol. 2022. PMID: 35911746 Free PMC article. Review.
-
Targeting TL1A and DR3: the new frontier of anti-cytokine therapy in IBD.Gut. 2025 Mar 6;74(4):652-668. doi: 10.1136/gutjnl-2024-332504. Gut. 2025. PMID: 39266053 Free PMC article. Review.
-
Analysis of therapeutic potential of preclinical models based on DR3/TL1A pathway modulation (Review).Exp Ther Med. 2021 Jul;22(1):693. doi: 10.3892/etm.2021.10125. Epub 2021 May 2. Exp Ther Med. 2021. PMID: 33986858 Free PMC article. Review.
-
Activation of DR3 signaling causes loss of ILC3s and exacerbates intestinal inflammation.Nat Commun. 2019 Jul 29;10(1):3371. doi: 10.1038/s41467-019-11304-8. Nat Commun. 2019. PMID: 31358760 Free PMC article.
-
ILC3: a case of conflicted identity.Front Immunol. 2023 Oct 17;14:1271699. doi: 10.3389/fimmu.2023.1271699. eCollection 2023. Front Immunol. 2023. PMID: 37915588 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

