Systemic Lupus Erythematosus: Definitions, Contexts, Conflicts, Enigmas
- PMID: 29545801
- PMCID: PMC5839091
- DOI: 10.3389/fimmu.2018.00387
Systemic Lupus Erythematosus: Definitions, Contexts, Conflicts, Enigmas
Abstract
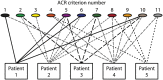
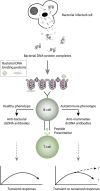
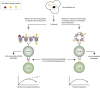
Systemic lupus erythematosus (SLE) is an inadequately defined syndrome. Etiology and pathogenesis remain largely unknown. SLE is on the other hand a seminal syndrome that has challenged immunologists, biologists, genetics, and clinicians to solve its nature. The syndrome is characterized by multiple, etiologically unlinked manifestations. Unexpectedly, they seem to occur in different stochastically linked clusters, although single gene defects may promote a smaller spectrum of symptoms/criteria typical for SLE. There is no known inner coherence of parameters (criteria) making up the disease. These parameters are, nevertheless, implemented in The American College of Rheumatology (ACR) and The Systemic Lupus Collaborating Clinics (SLICC) criteria to classify SLE. Still, SLE is an abstraction since the ACR or SLICC criteria allow us to define hundreds of different clinical SLE phenotypes. This is a major point of the present discussion and uses "The anti-dsDNA antibody" as an example related to the problematic search for biomarkers for SLE. The following discussion will show how problematic this is: the disease is defined through non-coherent classification criteria, its complexity is recognized and accepted, its pathogenesis is plural and poorly understood. Therapy is focused on dominant symptoms or organ manifestations, and not on the syndrome itself. From basic scientific evidences, we can add substantial amount of data that are not sufficiently considered in clinical medicine, which may change the paradigms linked to what "The Anti-DNA antibody" is-and is not-in context of the imperfectly defined syndrome SLE.
Keywords: anti-dsDNA antibodies; criteria; definitions; enigma; syndrome; systemic lupus erythematosus.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

