The Autoimmune Skin Disease Bullous Pemphigoid: The Role of Mast Cells in Autoantibody-Induced Tissue Injury
- PMID: 29545809
- PMCID: PMC5837973
- DOI: 10.3389/fimmu.2018.00407
The Autoimmune Skin Disease Bullous Pemphigoid: The Role of Mast Cells in Autoantibody-Induced Tissue Injury
Abstract
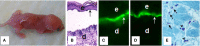
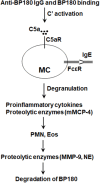
Bullous pemphigoid (BP) is an autoimmune and inflammatory skin disease associated with subepidermal blistering and autoantibodies directed against the hemidesmosomal components BP180 and BP230. Animal models of BP were developed by passively transferring anti-BP180 IgG into mice, which recapitulates the key features of human BP. By using these in vivo model systems, key cellular and molecular events leading to the BP disease phenotype are identified, including binding of pathogenic IgG to its target, complement activation of the classical pathway, mast cell degranulation, and infiltration and activation of neutrophils. Proteinases released by infiltrating neutrophils cleave BP180 and other hemidesmosome-associated proteins, causing DEJ separation. Mast cells and mast cell-derived mediators including inflammatory cytokines and proteases are increased in lesional skin and blister fluids of BP. BP animal model evidence also implicates mast cells in the pathogenesis of BP. However, recent studies questioned the pathogenic role of mast cells in autoimmune diseases such as multiple sclerosis, rheumatoid arthritis, and epidermolysis bullosa acquisita. This review highlights the current knowledge on BP pathophysiology with a focus on a potential role for mast cells in BP and mast cell-related critical issues needing to be addressed in the future.
Keywords: autoantibodies; bullous pemphigoid; hemidesmosome; mast cells; skin autoimmunity.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

