GEMALS: A promising therapy for amyotrophic lateral sclerosis
- PMID: 29545836
- PMCID: PMC5841048
- DOI: 10.3892/etm.2018.5868
GEMALS: A promising therapy for amyotrophic lateral sclerosis
Abstract
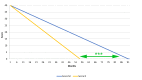
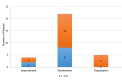
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that currently has no cure. At present, the only approved treatment for ALS is Riluzole, a glutamate release blocker that improves life expectancy by 3-6 months. ALS-Endotherapia (GEMALS) is a novel therapeutic approach to treat ALS and the aim of the present study was to investigate the potential beneficial effects of this novel treatment. A total of 31 patients with ALS were assessed in the current study. Deceleration of the disease was observed in 83.87% (P<0.0001) of patients and mean life expectancy was increased by 38 months. Motor functions, including breathing, walking, salivation, speech, swallowing and writing, were also improved in patients treated with GEMALS. The results of the present study demonstrate that long-term treatment with GEMALS has a curative effect in patients with ALS. Furthermore, the overall effectiveness of GEMALS was assessed using the ALS Assessment Questionnaire. The score improvement was 76.2 and 100% for men and women, respectively (P<0.0001), compared with the worldwide reference score. The present study provides a promising basis for the use of GEMALS as a therapeutic treatment for patients with ALS; however, these results must be confirmed in a double-blinded and randomized clinical trial.
Keywords: amyotrophic lateral sclerosis; endotherapia; human; poly-L-lysine.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
