Effect of TLR4/MyD88 signaling pathway on sepsis-associated acute respiratory distress syndrome in rats, via regulation of macrophage activation and inflammatory response
- PMID: 29545858
- PMCID: PMC5841028
- DOI: 10.3892/etm.2018.5815
Effect of TLR4/MyD88 signaling pathway on sepsis-associated acute respiratory distress syndrome in rats, via regulation of macrophage activation and inflammatory response
Abstract
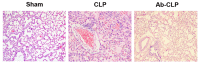
The present study aimed to investigate the effects of the Toll-like receptor (TLR)4/myeloid differentiation primary response (MyD)88 signaling pathway on sepsis-associated acute respiratory distress syndrome (ARDS) in rats, and the involvement of macrophage activation and the inflammatory response. A total of 36 specific pathogen-free male Sprague-Dawley rats were selected to establish the rat model of sepsis-associated ARDS using cecal ligation and puncture (CLP). Rats were assigned into the Ab (anti-TLR4 monoclonal antibody)-CLP, CLP and Sham groups. Arterial partial pressure of oxygen (PaO2) was detected using blood gas analysis. Bronchoalveolar lavage fluid (BALF) and alveolar macrophages were collected. The pathological structure of lung tissue was observed following hematoxylin-eosin staining. The ultrastructural alterations of alveolar epithelial cells were observed under transmission electron microscope. The ratios of wet/dry weight of lung tissue and total protein content in BALF were measured. The concentration of tumor necrosis factor (TNF)-α and interleukin (IL)-1β in BALF and peripheral blood was determined by enzyme-linked immunosorbent assay. The TLR4, TLR9, MyD88 and nuclear factor (NF)-κΒ mRNA and protein expression levels in alveolar macrophages were measured by reverse transcription-quantitative polymerase chain reaction and western blotting. Compared with the Sham group, the rats in the CLP group demonstrated significantly increased respiratory frequency, lung permeability, lung edema, inflammatory infiltration, TNF-α and IL-1β expression levels in BALF and peripheral blood and TLR4, TLR9, MyD88 and NF-κΒ expression levels in macrophages, however decreased arterial PaO2. Following pretreatment with anti-TLR4 monoclonal antibody, rats exhibited decreased lung injury, inflammatory infiltration, lung edema, TNF-α and IL-1β expressions in BALF and peripheral blood, and TLR4, TLR9, MyD88 and NF-κΒ expression levels in macrophages, with increased arterial PaO2. These results suggested that the inhibition of TLR4/MyD88 signaling pathway may relieve sepsis-associated ARDS in rats through regulating macrophage activation and the inflammatory response.
Keywords: TLR4/MyD88 signaling pathway; acute respiratory distress syndrome; inflammatory response; macrophage activation; sepsis.
Figures



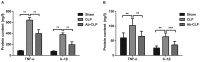


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
