5-FU therapeutic drug monitoring as a valuable option to reduce toxicity in patients with gastrointestinal cancer
- PMID: 29545919
- PMCID: PMC5837758
- DOI: 10.18632/oncotarget.24338
5-FU therapeutic drug monitoring as a valuable option to reduce toxicity in patients with gastrointestinal cancer
Abstract
Aims: 5-FU is used as the main backbone of chemotherapy regimens for patients with colorectal and other gastrointestinal cancers. Despite development of new strategies that allowed enhancing clinical effectiveness and tolerability of 5-FU, 10-30% of patients treated with 5-FU-based regimens experience severe treatment-related toxicity. In our study, we evaluated the 5-FU exposure-toxicity relationship and investigated the efficacy of PK-guided dosing in increasing tolerability of 5-FU-based chemotherapy.
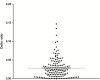
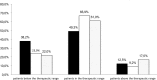
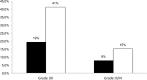
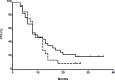
Results: 50.7% of patients required dose adjustments after cycle 1. Percentage of patients within 5-FU AUC range was 49.3%, 66.9%, 61.0% at cycle 1, 2 and 3 respectively (p = 0.002 cycle 1 vs cycle 2). At all 3 cycles, lower incidences of grade I/II toxicities were observed for patients below or within range compared with those above range (19.4% vs 41.3%, p < 0.001 respectively).
Conclusions: Our analysis confirms that the use of BSA-guided dosing results in highly variable 5-FU exposure and strongly suggests that PK-guided dosing can improve tolerability of 5-FU based chemotherapy in patients with gastrointestinal cancers, thus supporting 5-FU therapeutic drug monitoring.
Methods: 155 patients with gastrointestinal cancers, who were to receive 5-FU-based regimens were included in our study. At cycle 1, the 5-FU dose was calculated using patient's Body Surface Area (BSA) method. A blood sample was drawn on Day 2 to measure 5-FU concentration. At cycle 2, the 5-FU dose was adjusted using a PK-guided dosing strategy targeting a plasma AUC range of 18-28 mg·h/L, based on cycle 1 concentration. Assessments of toxicity was performed at the beginning of every cycle.
Keywords: 5-FU; GI cancer; adverse event; pharmacokinetics; therapeutic drug monitoring.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures

Similar articles
-
Therapeutic drug monitoring as a tool to optimize 5-FU-based chemotherapy in gastrointestinal cancer patients older than 75 years.Eur J Cancer. 2019 Apr;111:116-125. doi: 10.1016/j.ejca.2019.01.102. Epub 2019 Mar 5. Eur J Cancer. 2019. PMID: 30849685
-
A community-based multicenter trial of pharmacokinetically guided 5-fluorouracil dosing for personalized colorectal cancer therapy.Oncologist. 2014 Sep;19(9):959-65. doi: 10.1634/theoncologist.2014-0132. Epub 2014 Aug 12. Oncologist. 2014. PMID: 25117066 Free PMC article. Clinical Trial.
-
Individual fluorouracil dose adjustment in FOLFOX based on pharmacokinetic follow-up compared with conventional body-area-surface dosing: a phase II, proof-of-concept study.Clin Colorectal Cancer. 2012 Dec;11(4):263-7. doi: 10.1016/j.clcc.2012.05.004. Epub 2012 Jun 9. Clin Colorectal Cancer. 2012. PMID: 22683364 Clinical Trial.
-
Therapeutic drug monitoring of 5-fluorouracil.Cancer Chemother Pharmacol. 2016 Sep;78(3):447-64. doi: 10.1007/s00280-016-3054-2. Epub 2016 May 23. Cancer Chemother Pharmacol. 2016. PMID: 27217046 Free PMC article. Review.
-
How can we best monitor 5-FU administration to maximize benefit to risk ratio?Expert Opin Drug Metab Toxicol. 2018 Dec;14(12):1303-1313. doi: 10.1080/17425255.2018.1550484. Epub 2018 Nov 23. Expert Opin Drug Metab Toxicol. 2018. PMID: 30451549 Review.
Cited by
-
New insights into the mechanisms underlying 5-fluorouracil-induced intestinal toxicity based on transcriptomic and metabolomic responses in human intestinal organoids.Arch Toxicol. 2021 Aug;95(8):2691-2718. doi: 10.1007/s00204-021-03092-2. Epub 2021 Jun 20. Arch Toxicol. 2021. PMID: 34151400 Free PMC article.
-
Cardiotoxicity Associated With a Low Doses of 5-FU Promotes Morphoquantitative Changes in the Intrinsic Cardiac Nervous System.Cardiovasc Toxicol. 2025 Feb;25(2):193-204. doi: 10.1007/s12012-024-09958-y. Epub 2025 Jan 26. Cardiovasc Toxicol. 2025. PMID: 39864046
-
Therapeutic Drug Monitoring by Pharmacists: Does It Reduce Costs.Glob J Qual Saf Healthc. 2020 May 21;3(2):69-71. doi: 10.36401/JQSH-19-40. eCollection 2020 May. Glob J Qual Saf Healthc. 2020. PMID: 37334151 Free PMC article.
-
Nerolidol, Bioactive Compound Suppress Growth of HCT-116 Colorectal Cancer Cells Through Cell Cycle Arrest and Induction of Apoptosis.Appl Biochem Biotechnol. 2024 Mar;196(3):1365-1375. doi: 10.1007/s12010-023-04612-9. Epub 2023 Jul 3. Appl Biochem Biotechnol. 2024. PMID: 37395945
-
Case report: severe toxicity in an African-American patient receiving FOLFOX carrying uncommon allelic variants in DPYD.Pharmacogenomics. 2021 Jan;22(2):81-85. doi: 10.2217/pgs-2020-0120. Epub 2020 Dec 11. Pharmacogenomics. 2021. PMID: 33305610 Free PMC article.
References
-
- Capitain O, Boisdron-Celle M, Poirier AL, Abadie-Lacourtoisie S, Morel A, Gamelin E. The influence of fluorouracil outcome parameters on tolerance and efficacy in patients with advanced colorectal cancer. Pharmacogenomics J. 2008;8:256–67. https://doi.org/10.1038/sj.tpj.6500476. - DOI - PubMed
-
- Freeman K, Connock M, Cummins E, Gurung T, Taylor-Phillips S, Court R, Saunders M, Clarke A, Sutcliffe P. Fluorouracil plasma monitoring: systematic review and economic evaluation of the My5-FU assay for guiding dose adjustment in patients receiving fluorouracil chemotherapy by continuous infusion. Health Technol Assess. 2015;19:1–321. v–vi. v–vi. https://doi.org/10.3310/hta19910. - DOI - PMC - PubMed
-
- Lee JJ, Beumer JH, Chu E. Therapeutic drug monitoring of 5-fluorouracil. Cancer Chemother Pharmacol. 2016;78:447–64. https://doi.org/10.1007/s00280-016-3054-2. - DOI - PMC - PubMed
-
- Gamelin E, Boisdron-Celle M. Dose monitoring of 5-fluorouracil in patients with colorectal or head and neck cancer—status of the art. Crit Rev Oncol Hematol. 1999;30:71–79. https://doi.org/10.1016/S1040-8428(98)00036-5. - DOI - PubMed
-
- Tsalic M, Bar-Sela G, Beny A, Visel B, Haim N. Severe toxicity related to the 5-fluorouracil/leucovorin combination (the Mayo Clinic regimen): a prospective study in colorectal cancer patients. Am J Clin Oncol. 2003;26:103–06. https://doi.org/10.1097/01.COC.0000017526.55135.6D. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

