Sebinger Culture: A System Optimized for Morphological Maturation and Imaging of Cultured Mouse Metanephric Primordia
- PMID: 29546231
- PMCID: PMC5849297
- DOI: 10.21769/BioProtoc.2730
Sebinger Culture: A System Optimized for Morphological Maturation and Imaging of Cultured Mouse Metanephric Primordia
Abstract
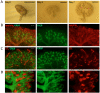
Here, we present a detailed protocol on setting up embryonic renal organ cultures using a culture method that we have optimised for anatomical maturation and imaging. Our culture method places kidney rudiments on glass in a thin film of medium, which results in very flat cultures with all tubules in the same image plane. For reasons not yet understood, this technique results in improved renal maturation compared to traditional techniques. Typically, this protocol will result in an organ formed with distinct cortical and medullary regions as well as elongated, correctly positioned loops of Henle. This article describes our method and provides detailed advice. We have published qualitative and quantitative evaluations on the performance of the technique in Sebinger et al. (2010) and Chang and Davies (2012).
Keywords: Imaging; Kidney; Metanephros; Organ culture; Organoid; Sebinger culture.
Figures



References
-
- Carrel A. and Burrows M. T.(1910). Cultivation of adult tissues and organs outside the body. J Am Med Ass 55: 1379-1381.
-
- Chang C. H. and Davies J. A.(2012). An improved method of renal tissue engineering, by combining renal dissociation and reaggregation with a low-volume culture technique, results in development of engineered kidneys complete with loops of Henle. Nephron Exp Nephrol 121(3-4): e79-85. - PubMed
-
- Davies J. A.(2006). A method for cold storage and transport of viable embryonic kidney rudiments. Kidney Int 70(11): 2031-2034. - PubMed
-
- Davies J. A., Ladomery M., Hohenstein P., Michael L., Shafe A., Spraggon L. and Hastie N.(2012). Development of an siRNA-based method for repressing specific genes in renal organ culture and its use to show that the Wt1 tumour suppressor is required for nephron differentiation. Hum Mol Genet 2004: 235-46. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

