Personalized Imaging and Modeling Strategies for Arrhythmia Prevention and Therapy
- PMID: 29546250
- PMCID: PMC5847279
- DOI: 10.1016/j.cobme.2017.11.007
Personalized Imaging and Modeling Strategies for Arrhythmia Prevention and Therapy
Abstract
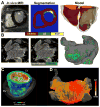
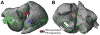
The goal of this article is to review advances in computational modeling of the heart, with a focus on recent non-invasive clinical imaging- and simulation-based strategies aimed at improving the diagnosis and treatment of patients with arrhythmias and structural heart disease. Following a brief overview of the field of computational cardiology, we present recent applications of the personalized virtual-heart approach in predicting the optimal targets for infarct-related ventricular tachycardia and atrial fibrillation ablation, and in determining risk of sudden cardiac death in myocardial infarction patients. The hope is that with such models at the patient bedside, therapies could be improved, invasiveness of diagnostic procedures minimized, and health-care costs reduced.
Figures


Similar articles
-
Computational modeling of cardiac electrophysiology and arrhythmogenesis: toward clinical translation.Physiol Rev. 2024 Jul 1;104(3):1265-1333. doi: 10.1152/physrev.00017.2023. Epub 2023 Dec 28. Physiol Rev. 2024. PMID: 38153307 Free PMC article. Review.
-
Advanced electrophysiologic mapping systems: an evidence-based analysis.Ont Health Technol Assess Ser. 2006;6(8):1-101. Epub 2006 Mar 1. Ont Health Technol Assess Ser. 2006. PMID: 23074499 Free PMC article.
-
How personalized heart modeling can help treatment of lethal arrhythmias: A focus on ventricular tachycardia ablation strategies in post-infarction patients.Wiley Interdiscip Rev Syst Biol Med. 2020 May;12(3):e1477. doi: 10.1002/wsbm.1477. Epub 2020 Jan 9. Wiley Interdiscip Rev Syst Biol Med. 2020. PMID: 31917524 Free PMC article. Review.
-
Interventional electrophysiology and its role in the treatment of cardiac arrhythmia.Ann Acad Med Singap. 1998 Mar;27(2):248-54. Ann Acad Med Singap. 1998. PMID: 9663319 Review.
-
Promising Therapies for Atrial Fibrillation and Ventricular Tachycardia.Int J Mol Sci. 2022 Oct 20;23(20):12612. doi: 10.3390/ijms232012612. Int J Mol Sci. 2022. PMID: 36293490 Free PMC article. Review.
Cited by
-
Semi-implicit Non-conforming Finite-Element Schemes for Cardiac Electrophysiology: A Framework for Mesh-Coarsening Heart Simulations.Front Physiol. 2018 Oct 30;9:1513. doi: 10.3389/fphys.2018.01513. eCollection 2018. Front Physiol. 2018. PMID: 30425648 Free PMC article.
-
Etiology-Specific Remodeling in Ventricular Tissue of Heart Failure Patients and Its Implications for Computational Modeling of Electrical Conduction.Front Physiol. 2021 Oct 5;12:730933. doi: 10.3389/fphys.2021.730933. eCollection 2021. Front Physiol. 2021. PMID: 34675817 Free PMC article.
-
A Computational Framework to Benchmark Basket Catheter Guided Ablation in Atrial Fibrillation.Front Physiol. 2018 Sep 21;9:1251. doi: 10.3389/fphys.2018.01251. eCollection 2018. Front Physiol. 2018. PMID: 30298012 Free PMC article.
-
Computational modeling of cardiac electrophysiology and arrhythmogenesis: toward clinical translation.Physiol Rev. 2024 Jul 1;104(3):1265-1333. doi: 10.1152/physrev.00017.2023. Epub 2023 Dec 28. Physiol Rev. 2024. PMID: 38153307 Free PMC article. Review.
-
From evidence-based medicine to digital twin technology for predicting ventricular tachycardia in ischaemic cardiomyopathy.J R Soc Interface. 2022 Sep;19(194):20220317. doi: 10.1098/rsif.2022.0317. Epub 2022 Sep 21. J R Soc Interface. 2022. PMID: 36128708 Free PMC article.
References
-
- Sugiura S, Washio T, Hatano A, Okada J, Watanabe H, Hisada T. Multi-scale simulations of cardiac electrophysiology and mechanics using the University of Tokyo heart simulator. Prog Biophys Mol Biol. 2012;110:380–389. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
