Anemia of Inflammation during Human Pregnancy Does Not Affect Newborn Iron Endowment
- PMID: 29546300
- PMCID: PMC6454452
- DOI: 10.1093/jn/nxx052
Anemia of Inflammation during Human Pregnancy Does Not Affect Newborn Iron Endowment
Abstract
Background: To our knowledge, no studies have addressed whether maternal anemia of inflammation (AI) affects newborn iron status, and few have addressed risk factors for specific etiologies of maternal anemia.
Objectives: The study aims were to evaluate 1) the contribution of AI and iron deficiency anemia (IDA) to newborn iron endowment, 2) hepcidin as a biomarker to distinguish AI from IDA among pregnant women, and 3) risk factors for specific etiologies of maternal anemia.
Methods: We measured hematologic biomarkers in maternal blood at 12 and 32 wk of gestation and in cord blood from a randomized trial of praziquantel in 358 pregnant women with Schistosoma japonicum in The Philippines. IDA was defined as anemia with serum ferritin <30 ng/mL and non-IDA (NIDA), largely due to AI, as anemia with ferritin ≥30 ng/mL. We identified cutoffs for biomarkers to distinguish IDA from NIDA by using area under the curve (AUC) analyses and examined the impact of different causes of anemia on newborn iron status (primary outcome) by using multivariate regression modeling.
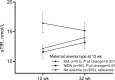
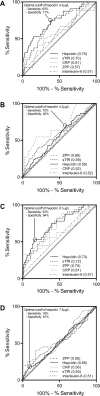
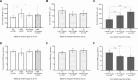
Results: Of the 358 mothers, 38% (n = 136) had IDA and 9% (n = 32) had NIDA at 32 wk of gestation. At 32 wk of gestation, serum hepcidin performed better than soluble transferrin receptor (sTfR) in identifying women with NIDA compared with the rest of the cohort (AUCs: 0.75 and 0.70, respectively) and in identifying women with NIDA among women with anemia (0.73 and 0.72, respectively). The cutoff that optimally distinguished women with NIDA from women with IDA in our cohort was 6.1 µg/L. Maternal IDA, but not NIDA, was associated with significantly lower newborn ferritin (114.4 ng/mL compared with 148.4 µg/L; P = 0.042).
Conclusions: Hepcidin performed better than sTfR in identifying pregnant women with NIDA, but its cost may limit its use. Maternal IDA, but not NIDA, is associated with decreased newborn iron stores, emphasizing the need to identify this cause and provide iron therapy. This trial was registered at www.clinicaltrials.gov as NCT00486863.
Figures

Similar articles
-
Serum Hepcidin Concentrations Decline during Pregnancy and May Identify Iron Deficiency: Analysis of a Longitudinal Pregnancy Cohort in The Gambia.J Nutr. 2017 Jun;147(6):1131-1137. doi: 10.3945/jn.116.245373. Epub 2017 Apr 19. J Nutr. 2017. PMID: 28424258 Free PMC article. Clinical Trial.
-
Iron deficiency and anemia are prevalent in women with multiple gestations.Am J Clin Nutr. 2016 Oct;104(4):1052-1060. doi: 10.3945/ajcn.115.126284. Epub 2016 Aug 31. Am J Clin Nutr. 2016. PMID: 27581469
-
Relationships between Maternal Obesity and Maternal and Neonatal Iron Status.Nutrients. 2018 Jul 30;10(8):1000. doi: 10.3390/nu10081000. Nutrients. 2018. PMID: 30061547 Free PMC article.
-
Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease.Nutrients. 2018 Aug 27;10(9):1173. doi: 10.3390/nu10091173. Nutrients. 2018. PMID: 30150549 Free PMC article. Review.
-
Adjusting total body iron for inflammation: Biomarkers Reflecting Inflammation and Nutritional Determinants of Anemia (BRINDA) project.Am J Clin Nutr. 2017 Jul;106(Suppl 1):383S-389S. doi: 10.3945/ajcn.116.142307. Epub 2017 Jun 14. Am J Clin Nutr. 2017. PMID: 28615255 Free PMC article. Review.
Cited by
-
Iron transport across the human placenta is regulated by hepcidin.Pediatr Res. 2022 Aug;92(2):396-402. doi: 10.1038/s41390-020-01201-y. Epub 2020 Oct 17. Pediatr Res. 2022. PMID: 33069164 Free PMC article.
-
Maternal hepcidin determines embryo iron homeostasis in mice.Blood. 2020 Nov 5;136(19):2206-2216. doi: 10.1182/blood.2020005745. Blood. 2020. PMID: 32584957 Free PMC article.
-
Hemoglobin and hepcidin have good validity and utility for diagnosing iron deficiency anemia among pregnant women.Eur J Clin Nutr. 2020 May;74(5):708-719. doi: 10.1038/s41430-019-0512-z. Epub 2019 Oct 17. Eur J Clin Nutr. 2020. PMID: 31624364 Free PMC article.
-
The effect of iron supplementation on maternal iron deficiency anemia does not differ by baseline anemia type among Tanzanian pregnant women without severe iron deficiency anemia.Eur J Nutr. 2023 Mar;62(2):987-1001. doi: 10.1007/s00394-022-03029-0. Epub 2022 Nov 8. Eur J Nutr. 2023. PMID: 36344770 Free PMC article. Clinical Trial.
-
Maternal anemia type during pregnancy is associated with anemia risk among offspring during infancy.Pediatr Res. 2019 Sep;86(3):396-402. doi: 10.1038/s41390-019-0433-5. Epub 2019 May 26. Pediatr Res. 2019. PMID: 31129681 Free PMC article. Clinical Trial.
References
-
- Pasricha SR, Drakesmith H. Iron deficiency anemia: problems in diagnosis and prevention at the population level. Hematol Oncol Clin North Am 2016;30:309–25. - PubMed
-
- Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, McGarvey ST, Kurtis JD, Friedman JF. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines. Am J Clin Nutr 2006;83:371–9. - PubMed
-
- van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr 2000;72(Suppl):247S–56S. - PubMed
-
- Kung'u JK, Wright VJ, Haji HJ, Ramsan M, Goodman D, Tielsch JM, Bickle QD, Raynes JG, Stoltzfus RJ. Adjusting for the acute phase response is essential to interpret iron status indicators among young Zanzibari children prone to chronic malaria and helminth infections. J Nutr 2009;139:2124–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

