A case of duodenal malignant lymphoma presenting as acute pancreatitis: systemic lupus erythematosus and immunosuppressive therapy as risk factors
- PMID: 29546569
- PMCID: PMC6096942
- DOI: 10.1007/s12328-018-0848-2
A case of duodenal malignant lymphoma presenting as acute pancreatitis: systemic lupus erythematosus and immunosuppressive therapy as risk factors
Abstract
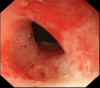
A 49-year-old man was admitted to our hospital with pancreatitis. He was diagnosed with systemic lupus erythematosus at 34 years of age and was being treated with oral tacrolimus (3 mg/day) and predonine (10 mg/day) for the past 15 months. The computed tomography (CT) scan showed the mass lesion had invaded the pancreatic head via thickening of the duodenal wall. Upper gastrointestinal endoscopy showed the all-round ulcerative lesion from the superior duodenal angle to the descending portion. Histological examination confirmed the diagnosis of diffuse large B cell lymphoma (DLBCL). Tacrolimus therapy was stopped due to the possibility of immunodeficiency-related lymphoproliferative disease; however, the lesion did not improve. Consequently, he was administered rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP). After six courses of R-CHOP therapy, a partial response was confirmed on CT. One month after the completion of chemotherapy, a gastrojejunal anastomosis was performed because of duodenal stenosis. He has since been well without recurrence. It was difficult to identify the risk factor for DLBCL; therefore, both the disease activity and immunosuppressive therapy should be taken into consideration as carrying a risk. In the present case, the symptom of pancreatitis enabled an early diagnosis of DLBCL.
Keywords: Duodenal malignant lymphoma; Immunosuppressive therapy-associated lymphoproliferative disorders; Pancreatitis; Systemic lupus erythematosus (SLE); Tacrolimus.
Conflict of interest statement
Conflict of interest
Reiko Yamada, Takashi Sakuno, Hiroyuki Inoue, Hiroshi Miura, Toshihumi Takeuchi, Yasunori Shiono, Hiroaki Okuse, Misaki Nakamura, Masaki Katsurahara, Yasuhiko Hamada, Kyosuke Tanaka, Noriyuki Horiki, and Yoshiyuki Takei declare that they have no conflict of interest.
Human rights
All procedures followed have been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

