Non-invasive brain stimulation techniques for chronic pain
- PMID: 29547226
- PMCID: PMC7039253
- DOI: 10.1002/14651858.CD008208.pub4
Non-invasive brain stimulation techniques for chronic pain
Update in
-
Non-invasive brain stimulation techniques for chronic pain.Cochrane Database Syst Rev. 2018 Apr 13;4(4):CD008208. doi: 10.1002/14651858.CD008208.pub5. Cochrane Database Syst Rev. 2018. PMID: 29652088 Free PMC article.
Abstract
Background: This is an updated version of the original Cochrane Review published in 2010, Issue 9, and last updated in 2014, Issue 4. Non-invasive brain stimulation techniques aim to induce an electrical stimulation of the brain in an attempt to reduce chronic pain by directly altering brain activity. They include repetitive transcranial magnetic stimulation (rTMS), cranial electrotherapy stimulation (CES), transcranial direct current stimulation (tDCS), transcranial random noise stimulation (tRNS) and reduced impedance non-invasive cortical electrostimulation (RINCE).
Objectives: To evaluate the efficacy of non-invasive cortical stimulation techniques in the treatment of chronic pain.
Search methods: For this update we searched CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, LILACS and clinical trials registers from July 2013 to October 2017.
Selection criteria: Randomised and quasi-randomised studies of rTMS, CES, tDCS, RINCE and tRNS if they employed a sham stimulation control group, recruited patients over the age of 18 years with pain of three months' duration or more, and measured pain as an outcome. Outcomes of interest were pain intensity measured using visual analogue scales or numerical rating scales, disability, quality of life and adverse events.
Data collection and analysis: Two review authors independently extracted and verified data. Where possible we entered data into meta-analyses, excluding studies judged as high risk of bias. We used the GRADE system to assess the quality of evidence for core comparisons, and created three 'Summary of findings' tables.
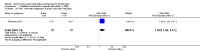
Main results: We included an additional 38 trials (involving 1225 randomised participants) in this update, making a total of 94 trials in the review (involving 2983 randomised participants). This update included a total of 42 rTMS studies, 11 CES, 36 tDCS, two RINCE and two tRNS. One study evaluated both rTMS and tDCS. We judged only four studies as low risk of bias across all key criteria. Using the GRADE criteria we judged the quality of evidence for each outcome, and for all comparisons as low or very low; in large part this was due to issues of blinding and of precision.rTMSMeta-analysis of rTMS studies versus sham for pain intensity at short-term follow-up (0 to < 1 week postintervention), (27 studies, involving 655 participants), demonstrated a small effect with heterogeneity (standardised mean difference (SMD) -0.22, 95% confidence interval (CI) -0.29 to -0.16, low-quality evidence). This equates to a 7% (95% CI 5% to 9%) reduction in pain, or a 0.40 (95% CI 0.53 to 0.32) point reduction on a 0 to 10 pain intensity scale, which does not meet the minimum clinically important difference threshold of 15% or greater. Pre-specified subgroup analyses did not find a difference between low-frequency stimulation (low-quality evidence) and rTMS applied to the prefrontal cortex compared to sham for reducing pain intensity at short-term follow-up (very low-quality evidence). High-frequency stimulation of the motor cortex in single-dose studies was associated with a small short-term reduction in pain intensity at short-term follow-up (low-quality evidence, pooled n = 249, SMD -0.38 95% CI -0.49 to -0.27). This equates to a 12% (95% CI 9% to 16%) reduction in pain, or a 0.77 (95% CI 0.55 to 0.99) point change on a 0 to 10 pain intensity scale, which does not achieve the minimum clinically important difference threshold of 15% or greater. The results from multiple-dose studies were heterogeneous and there was no evidence of an effect in this subgroup (very low-quality evidence). We did not find evidence that rTMS improved disability. Meta-analysis of studies of rTMS versus sham for quality of life (measured using the Fibromyalgia Impact Questionnaire (FIQ) at short-term follow-up demonstrated a positive effect (MD -10.80 95% CI -15.04 to -6.55, low-quality evidence).CESFor CES (five studies, 270 participants) we found no evidence of a difference between active stimulation and sham (SMD -0.24, 95% CI -0.48 to 0.01, low-quality evidence) for pain intensity. We found no evidence relating to the effectiveness of CES on disability. One study (36 participants) of CES versus sham for quality of life (measured using the FIQ) at short-term follow-up demonstrated a positive effect (MD -25.05 95% CI -37.82 to -12.28, very low-quality evidence).tDCSAnalysis of tDCS studies (27 studies, 747 participants) showed heterogeneity and a difference between active and sham stimulation (SMD -0.43 95% CI -0.63 to -0.22, very low-quality evidence) for pain intensity. This equates to a reduction of 0.82 (95% CI 0.42 to 1.2) points, or a percentage change of 17% (95% CI 9% to 25%) of the control group outcome. This point estimate meets our threshold for a minimum clinically important difference, though the lower confidence interval is substantially below that threshold. We found evidence of small study bias in the tDCS analyses. We did not find evidence that tDCS improved disability. Meta-analysis of studies of tDCS versus sham for quality of life (measured using different scales across studies) at short-term follow-up demonstrated a positive effect (SMD 0.66 95% CI 0.21 to 1.11, low-quality evidence).Adverse eventsAll forms of non-invasive brain stimulation and sham stimulation appear to be frequently associated with minor or transient side effects and there were two reported incidences of seizure, both related to the active rTMS intervention in the included studies. However many studies did not adequately report adverse events.
Authors' conclusions: There is very low-quality evidence that single doses of high-frequency rTMS of the motor cortex and tDCS may have short-term effects on chronic pain and quality of life but multiple sources of bias exist that may have influenced the observed effects. We did not find evidence that low-frequency rTMS, rTMS applied to the dorsolateral prefrontal cortex and CES are effective for reducing pain intensity in chronic pain. The broad conclusions of this review have not changed substantially for this update. There remains a need for substantially larger, rigorously designed studies, particularly of longer courses of stimulation. Future evidence may substantially impact upon the presented results.
Conflict of interest statement
NOC: none known
LM: none known
SS: none known
LHD: none known
BW: none known
Figures





























































Update of
-
Non-invasive brain stimulation techniques for chronic pain.Cochrane Database Syst Rev. 2014 Apr 11;(4):CD008208. doi: 10.1002/14651858.CD008208.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Mar 16;3:CD008208. doi: 10.1002/14651858.CD008208.pub4. PMID: 24729198 Updated.
References
References to studies included in this review
-
- Ahmed MA, Mohamed SA, Sayed D. Long‐term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta‐endorphin in patients with phantom pain. Neurological Research 2011;33(9):953‐8. - PubMed
-
- Ahn H, Wood, Adam J, Kunik ME, Bhattacharjee A, Chen Z, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: an experimenter‐ and participant‐blinded, randomized, sham‐controlled pilot clinical study. Brain Stimulation (in press). - PMC - PubMed
- Ahn H, Woods AJ, Choi E, Padhye N, Fillingim R. Transcranial direct current stimulation and mobility functioning in older adults with knee osteoarthritis pain: a double‐blind, randomized, sham‐controlled pilot clinical study. Brain Stimulation 2017;10:E21. - PMC - PubMed
-
- André‐Obadia N, Peyron R, Mertens P, Mauguière F, Laurent B, Garcia‐Larrea L. Transcranial magnetic stimulation for pain control. Double‐blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clinical Neurophysiology 2006;117(7):1536‐44. - PubMed
-
- André‐Obadia N, Mertens P, Gueguen A, Peyron R, Garcia‐Larrea L. Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology 2008;71(11):833‐40. - PubMed
-
- Andre‐Obadia N, Magnin M, Garcia‐Larrea L. On the importance of placebo timing in rTMS studies for pain relief. Pain 2011;152(6):1233‐7. - PubMed
References to studies excluded from this review
-
- Avery DH, Holtzheimer PE, Fawaz W, Russo J, Neumaier J, Dunner DL, et al. Transcranial magnetic stimulation reduces pain in patients with major depression: a sham‐controlled study. Journal of Nervous and Mental Disease 2007;195(5):378‐81. - PubMed
-
- Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004;42(7):417‐9. - PubMed
-
- Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain 2013;154(8):1274‐80. - PubMed
-
- Bolognini N, Spandri V, Ferraro F, Salmaggi A, Molinari ACL, Fregni F, et al. Immediate and sustained effects of 5‐day transcranial direct current stimulation of the motor cortex in phantom limb pain. Journal of Pain 2015;16(7):657‐65. - PubMed
-
- Carraro ER de O, Frazao ACDD, Soares KVB de C, Silva VF. Cerebral stimulation by photo and auditory synthesis associated to imagery and muscle therapy: reducing pain in women with fibromyalgia [Estimulação cerebral por sintetização fótica e auditiva associada àimagética e massoterapia: minimização de dor em mulheres portadoras de fibromialgia]. Motriz, Rio Claro 2010;16(2):359‐69.
References to studies awaiting assessment
-
- Acler M, Valenti D, Tocco P, Monaco S, Bertolasi L. Effects of non‐invasive cortical stimulation on fatigue and quality of life in post‐polio patients: a double blind real sham study. European Journal of Neurology 2012;19(Suppl 1):580.
-
- Albu S. Effectiveness of transcranial direct current stimulation in the treatment of chronic neuropathic pain in spinal cord injured patients. European Journal of Neurology 2011;18(Suppl 2):199.
-
- Fricova J, Klirova M, So P, Tilerova B, Masopust V, Hackel M, et al. Repetitive transcranial stimulation in chronic neurogenic pain. Pain Practice 2009;9(Suppl 1):38.
-
- Fricova J. Repetitive transcranial stimulation in chronic orofacial neurogenic pain treatment. Fundamental and Clinical Pharmacology 2011;25(Suppl 1):31.
-
- Fricová J, Klírová M, Masopust V, Novák T, Vérebová K, Rokyta R. Repetitive transcranial magnetic stimulation in the treatment of chronic orofacial pain. Physiological Research 2013;62 Suppl 1:S125‐34. - PubMed
References to ongoing studies
-
- ACTRN12612001155886. Investigating the role of transcranial direct current stimulation for pain relief in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome patients. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362490 (first received 14 May 2012).
-
- ACTRN12613000561785. Repetitive transcranial magnetic stimulation in the treatment of fibromyalgia [The effectiveness of repetitive transcranial magnetic stimulation in the treatment of fibromyalgia]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364236 (first received 13 May 2013).
-
- ACTRN12613001232729. Modulation of chronic pain perception with noninvasivecentral and peripheral nervous system stimulation [The effect of transcranial direct current stimulation and transcutaneous electrical nerve stimulation on improving pain intensity, physical functioning, mental health and quality of life in a chronic pain population awaiting pain clinic intervention]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365199 (first received 7 November 2013).
-
- ACTRN12614001247662. The effects of non‐invasive brain stimulation on chronic arm pain [The effects of non‐invasive brain stimulation on pain and the nociceptive system in people with chronic neuropathic pain]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367396 (first received 12 November 2014).
-
- ACTRN12615000110583. The impact of non‐invasive brain stimulation on motor cortex excitability and cognition in chronic lower back pain [In individuals with chronic lower back pain, does anodal transcranial direct current stimulation, compared to sham transcranial direct current stimulation, impact on motor cortex excitability and cognition?]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367643 (first received 27 January 2015).
Additional references
-
- Ahdab R, Ayache SS, Brugières P, Goujon C, Lefaucheur JP. Comparison of "standard" and "navigated" procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiologie Clinique 2010;40(1):27‐36. - PubMed
-
- Ambrus GG, Al‐Moyed H, Chaieb L, Sarp L, Antal A, Paulus W. The fade‐in‐short stimulation‐fade out approach to sham tDCS‐ reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimulation 2012;5(4):499‐504. - PubMed
-
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life and treatment. European Journal of Pain 2006;10:287‐333. - PubMed
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

