Copper-Catalyzed Vinylogous Aerobic Oxidation of Unsaturated Compounds with Air
- PMID: 29547276
- PMCID: PMC5927363
- DOI: 10.1021/jacs.8b01886
Copper-Catalyzed Vinylogous Aerobic Oxidation of Unsaturated Compounds with Air
Abstract
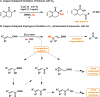
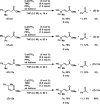
A mild and operationally simple copper-catalyzed vinylogous aerobic oxidation of β,γ- and α,β-unsaturated esters is described. This method features good yields, broad substrate scope, excellent chemo- and regioselectivity, and good functional group tolerance. This method is additionally capable of oxidizing β,γ- and α,β-unsaturated aldehydes, ketones, amides, nitriles, and sulfones. Furthermore, the present catalytic system is suitable for bisvinylogous and trisvinylogous oxidation. Tetramethylguanidine (TMG) was found to be crucial in its role as a base, but we also speculate that it serves as a ligand to copper(II) triflate to produce the active copper(II) catalyst. Mechanistic experiments conducted suggest a plausible reaction pathway via an allylcopper(II) species. Finally, the breadth of scope and power of this methodology are demonstrated through its application to complex natural product substrates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


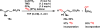
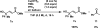


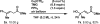
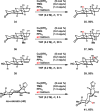
Similar articles
-
Expansion of Substrate Scope for Nitroxyl Radical/Copper-Catalyzed Aerobic Oxidation of Primary Alcohols: A Guideline for Catalyst Selection.Chem Pharm Bull (Tokyo). 2021;69(5):488-497. doi: 10.1248/cpb.c21-00043. Chem Pharm Bull (Tokyo). 2021. PMID: 33952858
-
A nickel catalyst for the addition of organoboronate esters to ketones and aldehydes.Org Lett. 2009 Oct 1;11(19):4410-3. doi: 10.1021/ol9017613. Org Lett. 2009. PMID: 19708680
-
Facile formation of β-hydroxyboronate esters by a Cu-catalyzed diboration/Matteson homologation sequence.Org Lett. 2014 Dec 5;16(23):6056-9. doi: 10.1021/ol502767m. Epub 2014 Nov 20. Org Lett. 2014. PMID: 25412356 Free PMC article.
-
CuH-catalyzed reactions.Chem Rev. 2008 Aug;108(8):2916-27. doi: 10.1021/cr0684321. Epub 2008 Jul 11. Chem Rev. 2008. PMID: 18616323 Review. No abstract available.
-
Asymmetric synthesis of tertiary alcohols and alpha-tertiary amines via Cu-catalyzed C-C bond formation to ketones and ketimines.Chem Rev. 2008 Aug;108(8):2853-73. doi: 10.1021/cr078340r. Epub 2008 Jun 21. Chem Rev. 2008. PMID: 18570481 Review. No abstract available.
Cited by
-
Chemoselektive γ-Oxidation von β,γ-ungesättigten Amiden mit TEMPO.Angew Chem Weinheim Bergstr Ger. 2021 Aug 23;133(35):19271-19275. doi: 10.1002/ange.202104023. Epub 2021 Jul 20. Angew Chem Weinheim Bergstr Ger. 2021. PMID: 38505148 Free PMC article.
-
Chemoselective γ-Oxidation of β,γ-Unsaturated Amides with TEMPO.Angew Chem Int Ed Engl. 2021 Aug 23;60(35):19123-19127. doi: 10.1002/anie.202104023. Epub 2021 Jul 20. Angew Chem Int Ed Engl. 2021. PMID: 34146371 Free PMC article.
-
Copper-Promoted Functionalization of Organic Molecules: from Biologically Relevant Cu/O2 Model Systems to Organometallic Transformations.Chem Rev. 2019 Feb 27;119(4):2954-3031. doi: 10.1021/acs.chemrev.8b00368. Epub 2019 Jan 30. Chem Rev. 2019. PMID: 30698952 Free PMC article. Review.
-
Acid-Catalyzed Air-Oxidative Fragmentation of the Carbon-Carbon Bond in 2-Aryl-1-tetralones.ACS Omega. 2019 May 2;4(5):8065-8070. doi: 10.1021/acsomega.9b00732. eCollection 2019 May 31. ACS Omega. 2019. PMID: 31459896 Free PMC article.
-
New Developments of the Principle of Vinylogy as Applied to π-Extended Enolate-Type Donor Systems.Chem Rev. 2020 Mar 11;120(5):2448-2612. doi: 10.1021/acs.chemrev.9b00481. Epub 2020 Feb 10. Chem Rev. 2020. PMID: 32040305 Free PMC article.
References
-
- Caron S, Dugger RW, Ruggeri SG, Ragan JA, Ripin DHB. Chem Rev. 2006;106:2943–2989. - PubMed
- Piera J, Bäckvall J-E. Angew Chem, Int Ed. 2008;47:3506–3523. - PubMed
- Cavani F, Teles JH. ChemSusChem. 2009;2:508–534. - PubMed
- Bäckvall J-E. Modern Oxidation Methods. 2nd. Wiley-VCH; Weinheim, Germany: 2011.
- Gunasekaran N. Adv Synth Catal. 2015;357:1990–2010.
-
- Stahl SS. Science. 2005;309:1824–1826. - PubMed
-
-
For some recent papers on O2 activation by transition metals, see:
- Garcia-Bosch I, Company A, Frisch JR, Torrent-Sucarrat M, Cardellach M, Gamba I, Güell M, Casella L, Que L, Ribas X, Luis JM, Costas M. Angew Chem, Int Ed. 2010;49:2406–2409. - PMC - PubMed
- Long R, Mao K, Ye X, Yan W, Huang Y, Wang J, Fu Y, Wang X, Wu X, Xie Y, Xiong Y. J Am Chem Soc. 2013;135:3200–3207. - PubMed
- Long R, Mao K, Gong M, Zhou S, Hu J, Zhi M, You Y, Bai S, Jiang J, Zhang Q, Wu X, Xiong Y. Angew Chem, Int Ed. 2014;53:3205–3209. - PubMed
- Liang YF, Jiao N. Acc Chem Res. 2017;50:1640–1653. - PubMed
-
-
- Foote CS, Valentine JS, Greenberg A, Liebman JF, editors. Active Oxygen in Chemistry. Blackie Academic & Professional; London: 1995.
-
-
For some recent reviews of transition-metal-catalyzed aerobic oxidation:
- Stahl SS. Angew Chem, Int Ed. 2004;43:3400–3420. - PubMed
- Punniyamurthy T, Velusamy S, Iqbal J. Chem Rev. 2005;105:2329–2363. - PubMed
- Sigman MS, Jensen DR. Acc Chem Res. 2006;39:221–229. - PubMed
- Boisvert L, Goldberg KI. Acc Chem Res. 2012;45:899–910. - PubMed
- Wu W, Jiang H. Acc Chem Res. 2012;45:1736–1748. - PubMed
- Shi Z, Zhang C, Tang C, Jiao N. Chem Soc Rev. 2012;41:3381–3430. - PubMed
- Roduner E, Kaim W, Sarkar B, Urlacher VB, Pleiss J, Gläser R, Einicke W-D, Sprenger GA, Beifuß U, Klemm E, Liebner C, Hieronymus H, Hsu S-F, Plietker B, Laschat S. ChemCatChem. 2013;5:82–112.
- Wang D, Weinstein AB, White PB, Stahl SS. Chem Rev. 2018;118:2636–2679. - PubMed
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

