Exfoliation Syndrome: A Disease of Autophagy and LOXL1 Proteopathy
- PMID: 29547474
- PMCID: PMC6028293
- DOI: 10.1097/IJG.0000000000000919
Exfoliation Syndrome: A Disease of Autophagy and LOXL1 Proteopathy
Abstract
Exfoliation syndrome (XFS) is an age-related disease involving the deposition of aggregated fibrillar material (exfoliation material) at extracellular matrices in tissues that synthesize elastic fibers. Its main morbidity is in the eye, where exfoliation material accumulations form on the surface of the ciliary body, iris, and lens. Exfoliation glaucoma (XFG) occurs in a high proportion of persons with XFS and can be a rapidly progressing disease. Worldwide, XFG accounts for about 25% of open-angle glaucoma cases. XFS and XFG show a sharp age-dependence, similarly to the many age-related diseases classified as aggregopathies. Progress in understanding the cellular bases for XFS/XFG has been slowed by a lack of experimental models. Working with primary human tenon fibroblasts (TF) derived from trabeculectomies of XFG patients and age-matched primary open-glaucoma controls, we found that TF from XFG cells display many of the functional features observed in cells from other protein aggregate diseases, such as Parkinson, Alzheimer, Huntington, and age-related macular degeneration. We have documented defects in lysosomal positioning, microtubule organization, autophagy processing rate, and mitochondrial health. In regard to failure of lysosomal and autophagosome positioning in XFG cells, we have found that XFG TF are unable to establish the transnuclear microtubule organizing center that is required for efficient centripetal vesicular locomotion along microtubules. In regard to potential sources of the autophagy malfunction, we have directed our attention to a potential role of the lysyl oxidase-like 1 protein (LOXL1), the elastic fiber catalyst that displays variant-dependent association with risk for XFG. Our experiments show that (a) in XFG cells, a substantial fraction of LOXL1 is processed for degradation by the autophagic system; (b) most of the LOXL1 N-terminus domain exists in a highly disordered state, a condition known to greatly increase the frequency of polypeptide misfolding; (c) that maximum misfolding occurs at amino acid position 153, the location of the high risk variant G153D; and (d) that replacement of glycine (G) by aspartate (D) there results in a substantial decrease in disorder within the 20 amino acid surrounding domain. Finally, we show that clusterin, a protein that can be induced by the presence of intracellular, or extracellular aggregates, is uniformly overexpressed in XFG TF. The implications of our results for a theory relating XFG to cellular aggregopathy are discussed.
Figures

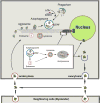
 ). γ-TuRCs, in turn, are densely packed by attachment to the cell centrosomes (
). γ-TuRCs, in turn, are densely packed by attachment to the cell centrosomes (
 ) via the adaptor protein ninein (NIN;
) via the adaptor protein ninein (NIN;
 ). Magnified MTOC detail: The insert depicts magnified details of the fusion events in the MTOC proximity. Early and late endosomes can fuse directly with lysosomes or with autophagasomes to form an amphisome. Both autophagosomes and amphisomes then fuse with the lysosome creating the autolysosome. In this transient structure the vesicular detritus cargo are lytically degraded into basic biochemical building blocks by the lysosome’s acidic hydrolases. Dense organelle accumulation at the MTOC greatly enhances these intervesicular fusions. Detritus that resists the sequential processing described above may accumulate in body inclusions (e.g., Lewy body in senile dementia), from where it may be exported. Re-uptake of these aggregates by the same (phagocytosis) will augment the load of undegredable protein in repetitive cycles. Furthermore, if exocytosis of aggregates or misfolded protein by the MAPS pathway described in Figure 1) is operative then the aggregopathy may propagate to adjacenct cells by particulate phagocytosis or endocytosis, as demonstrated for tau protein propagation in Alzheimer’s and α-synuclein protein in Parkinson’s.
). Magnified MTOC detail: The insert depicts magnified details of the fusion events in the MTOC proximity. Early and late endosomes can fuse directly with lysosomes or with autophagasomes to form an amphisome. Both autophagosomes and amphisomes then fuse with the lysosome creating the autolysosome. In this transient structure the vesicular detritus cargo are lytically degraded into basic biochemical building blocks by the lysosome’s acidic hydrolases. Dense organelle accumulation at the MTOC greatly enhances these intervesicular fusions. Detritus that resists the sequential processing described above may accumulate in body inclusions (e.g., Lewy body in senile dementia), from where it may be exported. Re-uptake of these aggregates by the same (phagocytosis) will augment the load of undegredable protein in repetitive cycles. Furthermore, if exocytosis of aggregates or misfolded protein by the MAPS pathway described in Figure 1) is operative then the aggregopathy may propagate to adjacenct cells by particulate phagocytosis or endocytosis, as demonstrated for tau protein propagation in Alzheimer’s and α-synuclein protein in Parkinson’s.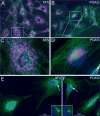

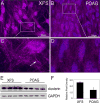
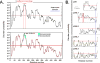
References
-
- Ritch R, Schlötzer-Schrehardt U. Exfoliation syndrome. Surv Ophthalmol. 2001;45:265–315. - PubMed
-
- Ovodenko B, Rostagno A, Neubert TA, et al. Proteomic analysis of exfoliation deposits. Invest Ophthalmol Vis Sci. 2007;48:1447–1457. - PubMed
-
- Schlötzer-Schrehardt U, Naumann GO. A histopathologic study of zonular instability in pseudoexfoliation syndrome. Am J Ophthalmol. 1994;118:730–743. - PubMed
-
- Schlötzer-Schrehardt U, Zenkel M, Küchle M, Sakai LY, Naumann GO. Role of transforming growth factor-beta1 and its latent form binding protein in pseudoexfoliation syndrome. Exp Eye Res. 2001;73:765–780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

