The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates
- PMID: 29547658
- PMCID: PMC5856377
- DOI: 10.1371/journal.pone.0194345
The corneal epitheliotrophic abilities of lyophilized powder form human platelet lysates
Abstract
Purpose: To evaluate whether lyophilized human platelet lysate (HPL) powder can preserve the growth factor concentrations and epitheliotrophic properties of liquid HPL, and potentially be used as a clinically-friendly treatment option.
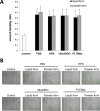
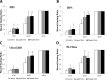
Methods: Two commercialized liquid HPLs, UltraGRO TM (Helios, Atlanta, GA) and PLTMax (Mill Creek, Rochester, MI), were obtained and converted to lyophilized powder. After redissolution, lyophilized powder HPLs were compared with liquid HPLs, as well as human peripheral serum (HPS) and fetal bovine serum (FBS) in liquid or redissolved lyophilized powder forms. Concentrations of epidermal growth factor (EGF), transforming growth factor-β1 (TGF-β1), platelet-derived growth factor-AB (PDGF-AB) and platelet-derived growth factor-BB (PDGF-BB) were evaluated by enzyme-linked immunosorbent assay (ELISA). Human corneal epithelial cell line was incubated with the blood derivatives and evaluated for cell migration with scratch-induced directional wounding and proliferation with MTS assays. Cell differentiation was examined by transepithelial electrical resistance (TEER). Fluorescein staining and in vivo confocal microscopy were used to evaluate in vivo corneal epithelial wound healing in Sprague-Dawley rats that underwent corneal debridement and topical application of liquid and redissolved powder HPLs.
Results: Liquid form and redissolved lyophilized powder form HPLs had similar concentrations of EGF, TGF-β1, PDGF-AB and PDGF-BB. In vitro experiments on cell migration, proliferation and differentiation and rat models on wound healing demonstrated no significant difference between the liquid and redissolved lyophilized powder forms for HPLs, HPS and FBS. In vivo confocal microscopy revealed similar wound healing process at different layers of cornea after corneal epithelial debridement between liquid form and redissolved lyophilized power form of HPLs.
Conclusions: The redissolved lyophilized powder form of both commercialized HPLs showed similar growth factor concentrations and corneal epitheliotrophic abilities compared to the liquid form. Results suggest that the properties of liquid HPLs can be retained despite lyophilization and that lyophilized HPLs can be a treatment option for corneal epithelial disorders.
Conflict of interest statement
Figures






Similar articles
-
Comparison of corneal epitheliotrophic capacities among human platelet lysates and other blood derivatives.PLoS One. 2017 Feb 2;12(2):e0171008. doi: 10.1371/journal.pone.0171008. eCollection 2017. PLoS One. 2017. PMID: 28152010 Free PMC article.
-
The effect of human platelet lysate on corneal nerve regeneration.Br J Ophthalmol. 2021 Jun;105(6):884-890. doi: 10.1136/bjophthalmol-2019-314408. Epub 2019 Nov 20. Br J Ophthalmol. 2021. PMID: 31748333
-
Corneal epitheliotrophic capacity of three different blood-derived preparations.Invest Ophthalmol Vis Sci. 2006 Jun;47(6):2438-44. doi: 10.1167/iovs.05-0876. Invest Ophthalmol Vis Sci. 2006. PMID: 16723454
-
Human platelet lysate delivered via an ocular wound chamber for the treatment of corneal epithelial injuries.Exp Eye Res. 2021 May;206:108493. doi: 10.1016/j.exer.2021.108493. Epub 2021 Feb 14. Exp Eye Res. 2021. PMID: 33596441 Review.
-
Corneal epithelial wound healing.Exp Biol Med (Maywood). 2001 Jul;226(7):653-64. doi: 10.1177/153537020222600711. Exp Biol Med (Maywood). 2001. PMID: 11444101 Review.
Cited by
-
Freeze-drying protocols and methods of maintaining the in-vitro biological activity of horse platelet lysate.Int J Vet Sci Med. 2024 Aug 7;12(1):71-80. doi: 10.1080/23144599.2024.2380586. eCollection 2024. Int J Vet Sci Med. 2024. PMID: 39119550 Free PMC article.
-
Cytokines in equine platelet lysate and related blood products.Front Vet Sci. 2023 Mar 9;10:1117829. doi: 10.3389/fvets.2023.1117829. eCollection 2023. Front Vet Sci. 2023. PMID: 36968472 Free PMC article.
-
Pigment Epithelium-Derived Factor Peptide Promotes Corneal Nerve Regeneration: An In Vivo and In Vitro Study.Invest Ophthalmol Vis Sci. 2021 Jan 4;62(1):23. doi: 10.1167/iovs.62.1.23. Invest Ophthalmol Vis Sci. 2021. PMID: 33481984 Free PMC article.
-
Human Platelet Lysate Treatment for Exposure Keratopathy: An In Vivo Confocal Microscopy and Anterior Segment OCT Study.Invest Ophthalmol Vis Sci. 2025 Jun 2;66(6):73. doi: 10.1167/iovs.66.6.73. Invest Ophthalmol Vis Sci. 2025. PMID: 40552916 Free PMC article.
-
Differences between the Proliferative Effects of Human Platelet Lysate and Fetal Bovine Serum on Human Adipose-Derived Stem Cells.Cells. 2019 Oct 8;8(10):1218. doi: 10.3390/cells8101218. Cells. 2019. PMID: 31597348 Free PMC article.
References
-
- Noda-Tsuruya T, Asano-Kato N, Toda I, Tsubota K. Autologous serum eye drops for dry eye after LASIK. Journal of refractive surgery. 2006;22(1):61–6. - PubMed
-
- Hartwig D, Herminghaus P, Wedel T, Liu L, Schlenke P, Dibbelt L, et al. Topical treatment of ocular surface defects: comparison of the epitheliotrophic capacity of fresh frozen plasma and serum on corneal epithelial cells in an in vitro cell culture model. Transfusion medicine. 2005;15(2):107–13. doi: 10.1111/j.0958-7578.2005.00559.x - DOI - PubMed
-
- Brown SM, Bradley JC. The effect of autologous serum eye drops in the treatment of severe dry eye disease: a prospective randomized case-control study. American journal of ophthalmology. 2005;140(3):565; author reply -6. doi: 10.1016/j.ajo.2005.03.067 - DOI - PubMed
-
- Matsumoto Y, Dogru M, Goto E, Ohashi Y, Kojima T, Ishida R, et al. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology. 2004;111(6):1115–20. doi: 10.1016/j.ophtha.2003.10.019 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

