CDK and MAPK Synergistically Regulate Signaling Dynamics via a Shared Multi-site Phosphorylation Region on the Scaffold Protein Ste5
- PMID: 29547722
- PMCID: PMC5858200
- DOI: 10.1016/j.molcel.2018.02.018
CDK and MAPK Synergistically Regulate Signaling Dynamics via a Shared Multi-site Phosphorylation Region on the Scaffold Protein Ste5
Abstract
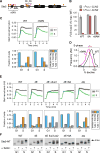
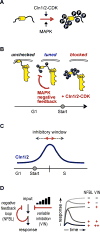
We report an unanticipated system of joint regulation by cyclin-dependent kinase (CDK) and mitogen-activated protein kinase (MAPK), involving collaborative multi-site phosphorylation of a single substrate. In budding yeast, the protein Ste5 controls signaling through a G1 arrest pathway. Upon cell-cycle entry, CDK inhibits Ste5 via multiple phosphorylation sites, disrupting its membrane association. Using quantitative time-lapse microscopy, we examined Ste5 membrane recruitment dynamics at different cell-cycle stages. Surprisingly, in S phase, where Ste5 recruitment should be blocked, we observed an initial recruitment followed by a steep drop-off. This delayed inhibition revealed a requirement for both CDK activity and negative feedback from the pathway MAPK Fus3. Mutagenesis, mass spectrometry, and electrophoretic analyses suggest that the CDK and MAPK modify shared sites, which are most extensively phosphorylated when both kinases are active and able to bind their docking sites on Ste5. Such collaborative phosphorylation can broaden regulatory inputs and diversify output dynamics of signaling pathways.
Keywords: Cdc28; Cks1; Cln2; G protein; Ste4; cyclin; mating; pheromone; signal transduction; start.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Alon U. An Introduction to Systems Biology: Design Principles of Biological Circuits. Chapman & Hall/CRC Mathematical & Computational Biology; Chapman & Hall; 2006.
-
- Bhattacharyya RP, Remenyi A, Good MC, Bashor CJ, Falick AM, Lim WA. The Ste5 scaffold allosterically modulates signaling output of the yeast mating pathway. Science. 2006;311:822–826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

