Lipid peroxidation regulates podocyte migration and cytoskeletal structure through redox sensitive RhoA signaling
- PMID: 29547847
- PMCID: PMC5854917
- DOI: 10.1016/j.redox.2018.02.024
Lipid peroxidation regulates podocyte migration and cytoskeletal structure through redox sensitive RhoA signaling
Abstract
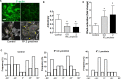
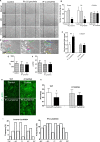
Early podocyte loss is characteristic of chronic kidney diseases (CKD) in obesity and diabetes. Since treatments for hyperglycemia and hypertension do not prevent podocyte loss, there must be additional factors causing podocyte depletion. The role of oxidative stress has been implicated in CKD but it is not known how exactly free radicals affect podocyte physiology. To assess this relationship, we investigated the effects of lipid radicals on podocytes, as lipid peroxidation is a major form of oxidative stress in diabetes. We found that lipid radicals govern changes in podocyte homeostasis through redox sensitive RhoA signaling: lipid radicals inhibit migration and cause loss of F-actin fibers. These effects were prevented by mutating the redox sensitive cysteines of RhoA. We therefore suggest that in diseases associated with increased lipid peroxidation, lipid radicals can determine podocyte function with potentially pathogenic consequences for kidney physiology.
Keywords: Chronic kidney disease; Cysteine; Lipid peroxidation; Podocyte; Reactive lipids; RhoA.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Role of γ-adducin in actin cytoskeleton rearrangements in podocyte pathophysiology.Am J Physiol Renal Physiol. 2021 Jan 1;320(1):F97-F113. doi: 10.1152/ajprenal.00423.2020. Epub 2020 Dec 14. Am J Physiol Renal Physiol. 2021. PMID: 33308016 Free PMC article.
-
RhoA deficiency disrupts podocyte cytoskeleton and induces podocyte apoptosis by inhibiting YAP/dendrin signal.BMC Nephrol. 2016 Jul 7;17(1):66. doi: 10.1186/s12882-016-0287-6. BMC Nephrol. 2016. PMID: 27389190 Free PMC article.
-
Advanced glycation end products inhibit adhesion ability of differentiated podocytes in a neuropilin-1-dependent manner.Am J Physiol Renal Physiol. 2011 Oct;301(4):F852-70. doi: 10.1152/ajprenal.00575.2010. Epub 2011 Jul 6. Am J Physiol Renal Physiol. 2011. PMID: 21734098
-
Actin dynamics at focal adhesions: a common endpoint and putative therapeutic target for proteinuric kidney diseases.Kidney Int. 2018 Jun;93(6):1298-1307. doi: 10.1016/j.kint.2017.12.028. Epub 2018 Apr 17. Kidney Int. 2018. PMID: 29678354 Free PMC article. Review.
-
Role of oxidized low density lipoproteins and free fatty acids in the pathogenesis of glomerulopathy and tubulointerstitial lesions in type 2 diabetes.Nutr Metab Cardiovasc Dis. 2011 Feb;21(2):79-85. doi: 10.1016/j.numecd.2010.10.002. Epub 2010 Dec 24. Nutr Metab Cardiovasc Dis. 2011. PMID: 21186102 Review.
Cited by
-
RIPK3 Contributes to Lyso-Gb3-Induced Podocyte Death.Cells. 2021 Jan 27;10(2):245. doi: 10.3390/cells10020245. Cells. 2021. PMID: 33513913 Free PMC article.
-
Alleviation by Mahuang Fuzi and Shenzhuo Decoction in High Glucose-Induced Podocyte Injury by Inhibiting the Activation of Wnt/β-Catenin Signaling Pathway, Resulting in Activation of Podocyte Autophagy.Evid Based Complement Alternat Med. 2020 Sep 3;2020:7809427. doi: 10.1155/2020/7809427. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32963573 Free PMC article.
-
Association of serum lycopene concentrations with all-cause and cardiovascular mortality among individuals with chronic kidney disease: A cohort study.Front Nutr. 2022 Dec 5;9:1048884. doi: 10.3389/fnut.2022.1048884. eCollection 2022. Front Nutr. 2022. PMID: 36545466 Free PMC article.
-
Type III intermediate filaments in redox interplay: key role of the conserved cysteine residue.Biochem Soc Trans. 2024 Apr 24;52(2):849-860. doi: 10.1042/BST20231059. Biochem Soc Trans. 2024. PMID: 38451193 Free PMC article. Review.
-
Role of γ-adducin in actin cytoskeleton rearrangements in podocyte pathophysiology.Am J Physiol Renal Physiol. 2021 Jan 1;320(1):F97-F113. doi: 10.1152/ajprenal.00423.2020. Epub 2020 Dec 14. Am J Physiol Renal Physiol. 2021. PMID: 33308016 Free PMC article.
References
-
- Sachs N., Sonnenberg A. Cell-matrix adhesion of podocytes in physiology and disease. Nat. Rev. Nephrol. 2013;9:200–210. - PubMed
-
- Barisoni L., Mundel P. Podocyte biology and the emerging understanding of podocyte diseases. Am. J. Nephrol. 2003;23:353–360. - PubMed
-
- Endlich K., Kriz W., Witzgall R. Update in podocyte biology. Curr. Opin. Nephrol. Hypertens. 2001;10:331–340. - PubMed
-
- Li J.J. Podocyte biology in diabetic nephropathy. Kidney Int. Suppl. 2007:S36–S42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

