Potential targets for next generation antimicrobial glycoconjugate vaccines
- PMID: 29547971
- PMCID: PMC5995208
- DOI: 10.1093/femsre/fuy011
Potential targets for next generation antimicrobial glycoconjugate vaccines
Abstract
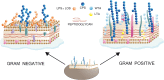
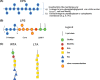
Cell surface carbohydrates have been proven optimal targets for vaccine development. Conjugation of polysaccharides to a carrier protein triggers a T-cell-dependent immune response to the glycan moiety. Licensed glycoconjugate vaccines are produced by chemical conjugation of capsular polysaccharides to prevent meningitis caused by meningococcus, pneumococcus and Haemophilus influenzae type b. However, other classes of carbohydrates (O-antigens, exopolysaccharides, wall/teichoic acids) represent attractive targets for developing vaccines. Recent analysis from WHO/CHO underpins alarming concern toward antibiotic-resistant bacteria, such as the so called ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp.) and additional pathogens such as Clostridium difficile and Group A Streptococcus. Fungal infections are also becoming increasingly invasive for immunocompromised patients or hospitalized individuals. Other emergencies could derive from bacteria which spread during environmental calamities (Vibrio cholerae) or with potential as bioterrorism weapons (Burkholderia pseudomallei and mallei, Francisella tularensis). Vaccination could aid reducing the use of broad-spectrum antibiotics and provide protection by herd immunity also to individuals who are not vaccinated.This review analyzes structural and functional differences of the polysaccharides exposed on the surface of emerging pathogenic bacteria, combined with medical need and technological feasibility of corresponding glycoconjugate vaccines.
Figures


References
-
- Adamo R. Advancing homogeneous antimicrobial glycoconjugate vaccines. Acc Chem Res 2017;50:1270–9. - PubMed
-
- Adamo R, Hu Q-Y, Torosantucci A et al. . Deciphering the structure–immunogenicity relationship of anti-Candida glycoconjugate vaccines. Chem Sci 2014;5:4302–11.
-
- Adamo R, Romano MR, Berti F et al. . Phosphorylation of the synthetic hexasaccharide repeating unit is essential for the induction of antibodies to Clostridium difficile PSII cell wall polysaccharide. ACS Chem Biol 2012;7:1420–8. - PubMed
-
- Adamo R, Tontini M, Brogioni G et al. . Synthesis of laminarin fragments and evaluation of a β-(1,3) glucan hexasaccharide-CRM 197 conjugate as vaccine candidate against Candida albicans. J Carbohydr Chem 2011;30:249–80.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

