Prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency among malaria patients in Upper Myanmar
- PMID: 29548282
- PMCID: PMC5857094
- DOI: 10.1186/s12879-018-3031-y
Prevalence of glucose-6-phosphate dehydrogenase (G6PD) deficiency among malaria patients in Upper Myanmar
Abstract
Background: Glucose-6-phosphate dehydrogenase (G6PD; EC 1.1.1.49) deficiency is one of the most common X-linked recessive hereditary disorders in the world. Primaquine (PQ) has been used for radical cure of P. vivax to prevent relapse. Recently, it is also used to reduce P. falciparum gametocyte carriage to block transmission. However, PQ metabolites oxidize hemoglobin and generate excessive reactive oxygen species which can trigger acute hemolytic anemia in malaria patients with inherited G6PD deficiency.
Methods: A total of 252 blood samples collected from malaria patients in Myanmar were used in this study. G6PD variant was analysed by a multiplex allele specific PCR kit, DiaPlexC™ G6PD Genotyping Kit [Asian type]. The accuracy of the multiplex allele specific PCR was confirmed by sequencing analysis.
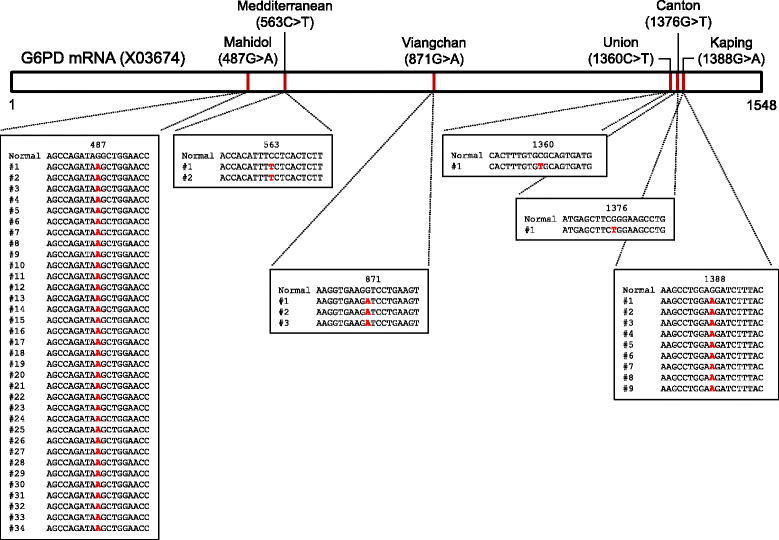
Results: Prevalence and distribution of G6PD variants in 252 malaria patients in Myanmar were analysed. Six different types of G6PD allelic variants were identified in 50 (7 females and 43 males) malaria patients. The predominant variant was Mahidol (68%, 34/50), of which 91.2% (31/34) and 8.8% (3/34) were males and females, respectively. Other G6PD variants including Kaiping (18%, 9/50), Viangchan (6%, 3/50), Mediterranean (4%, 2/50), Union (2%, 1/50) and Canton (2%, 1/50) were also observed.
Conclusions: Results of this study suggest that more concern for proper and safe use of PQ as a radical cure of malaria in Myanmar is needed by combining G6PD deficiency test before PQ prescription. Establishment of a follow-up system to monitor potential PQ toxicity in malaria patients who are given PQ is also required.
Keywords: G6PD deficiency; Glucose-6-phosphate dehydrogenase (G6PD); Malaria; Myanmar; Primaquine.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the Ethics Review Committee, Department of Medical Research, Myanmar (1/Ethics/DMRUM/2013 and 97/Ethics 2015), and by the Ethical Review Committee of Inha University School of Medicine, Korea (INHA 15–013). Informed written consent was obtained from each patient.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

