The combined effect of Parathyroid hormone (1-34) and whole-body Vibration exercise in the treatment of OSteoporosis (PaVOS)- study protocol for a randomized controlled trial
- PMID: 29548300
- PMCID: PMC5857123
- DOI: 10.1186/s13063-018-2551-5
The combined effect of Parathyroid hormone (1-34) and whole-body Vibration exercise in the treatment of OSteoporosis (PaVOS)- study protocol for a randomized controlled trial
Abstract
Background: PaVOS is a randomized controlled trial (RCT) which aims to address the use of whole-body vibration exercise (WBV) in combination with parathyroid hormone 1-34 fragment teriparatide (PTH 1-34) treatment in patients with osteoporosis. PTH 1-34 is an effective but expensive anabolic treatment for osteoporosis. WBV has been found to stimulate muscle and bone growth. Animal studies have shown a beneficial effect on bone when combining PTH 1-34 with mechanical loading. A combined treatment with PTH 1-34 and WBV may potentially have beneficial effects on bone and muscles, and reduce fracture risk.
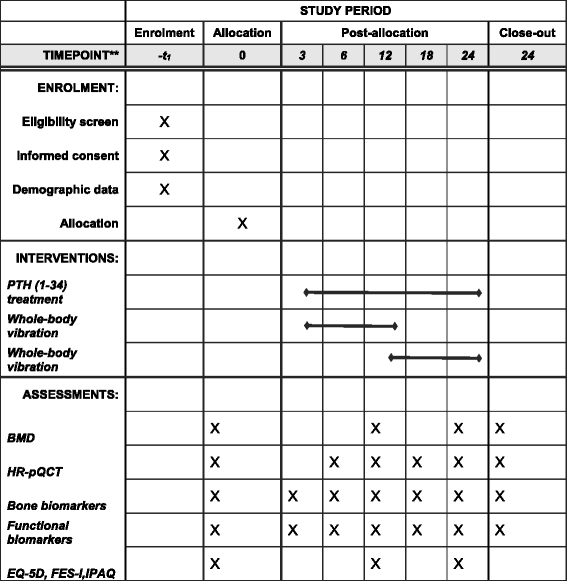
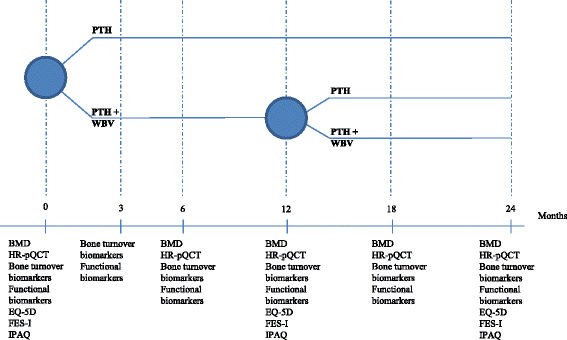
Methods/design: PaVOS is a multicenter, assessor-blinded, superiority, two-armed randomized controlled trial (RCT). Postmenopausal women (n = 40, aged 50 years and older) starting taking PTH 1-34 from outpatient clinics will be randomized and assigned to a PTH 1-34 + WBV-exercise group (intervention group), or a PTH 1-34-alone group (control group). The intervention group will undergo WBV three sessions a week (12 min each, including 1:1 ratio of exercise: rest, 30 Hz, 1 mm amplitude) for a 12-month intervention period. Both the intervention and the control group will receive PTH 1-34 treatment (20 μg s.c. daily) for 24 months. After 12 months the WBV group will be re-randomized to stop or continue WBV for an additional 12 months. The primary endpoint, bone mineral density (BMD), will be measured by dual-energy x-ray absorptiometry of the total hip and the lumbar spine. Secondary endpoints, bone microarchitecture and estimated bone strength, will be assessed using high-resolution peripheral quantitative computed tomography (HR-pQCT) of the radius and tibia. Serum bone turnover markers (carboxy-terminal collagen crosslinks (CTX), amino-terminal propeptide of type-I collagen (P1NP), and sclerostin) and functional biomarkers (Timed Up and Go (TUG), Short Physical Performance Battery (SPPB), grip strength, and leg extension power) will be measured to assess the effect on bone turnover, muscle strength, balance, and functionality. Quality of life (EQ-5D), physical activity (IPAQ) and fear of falling (FES-I) will be assessed by questionnaires. Data on adherence and falls incidence will be collected.
Discussion: The PaVOS study will investigate the effects of WBV in combination with PTH 1-34 on bone parameters in postmenopausal women.
Trial registration: ClinicalTrials.gov, ID: NCT02563353 . Registered on 30 September 2015.
Keywords: Bone mineral density; Bone quality; Osteoporosis; Parathyroid hormone; RCT; Teriparatide; Whole-body vibration.
Conflict of interest statement
Ethics approval and consent to participate
This study will be conducted according to the standards of International Conference on Harmonization, Research Ethics Committee regulations, any applicable government regulations (e.g., The Danish Data Protection Agency), and local procedures. The protocol is approved by the Regional Scientific Ethical Committee of Southern Denmark (ref. ID S-20150121) and The Danish Data Protection Agency (2008-58-0035, 13/40496). Any amendments will be submitted to the above ethical committee for approval of the study conduct. The participants are covered by the Danish “Complaint and compensation law in health care.” Written informed consent will be obtained from each participant. The trial is audited in an ongoing manner to insure patient safety. All events and adverse events are audited each year. In case of serious adverse events these will be sent for audit within 2 weeks. The events are audited independent from the trial investigators by The Regional Scientific Ethical Committee of Southern Denmark.
Consent for publication
Not applicable
Competing interests
The investigators have no competing interests in the study.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
The combined effect of parathyroid hormone (1-34) and whole-body vibration exercise on physical performance in OSteoporotic women (PaVOS study): a secondary analysis from a randomised controlled trial.BMC Sports Sci Med Rehabil. 2020 Sep 5;12:54. doi: 10.1186/s13102-020-00204-w. eCollection 2020. BMC Sports Sci Med Rehabil. 2020. PMID: 32944251 Free PMC article.
-
The combined effect of Parathyroid hormone (1-34) and whole-body Vibration exercise in the treatment of postmenopausal OSteoporosis (PaVOS study): a randomized controlled trial.Osteoporos Int. 2019 Sep;30(9):1827-1836. doi: 10.1007/s00198-019-05029-z. Epub 2019 Jul 15. Osteoporos Int. 2019. PMID: 31309239 Free PMC article. Clinical Trial.
-
Effect of 12 months of whole-body vibration therapy on bone density and structure in postmenopausal women: a randomized trial.Ann Intern Med. 2011 Nov 15;155(10):668-79, W205. doi: 10.7326/0003-4819-155-10-201111150-00005. Ann Intern Med. 2011. PMID: 22084333 Clinical Trial.
-
Therapeutic potential of parathyroid hormone.Curr Osteoporos Rep. 2004 Mar;2(1):5-11. doi: 10.1007/s11914-004-0008-0. Curr Osteoporos Rep. 2004. PMID: 16036076 Review.
-
Effects of parathyroid hormone alone or in combination with antiresorptive therapy on bone mineral density and fracture risk--a meta-analysis.Osteoporos Int. 2007 Jan;18(1):45-57. doi: 10.1007/s00198-006-0204-0. Epub 2006 Sep 2. Osteoporos Int. 2007. PMID: 16951908 Review.
Cited by
-
The combined effect of parathyroid hormone (1-34) and whole-body vibration exercise on physical performance in OSteoporotic women (PaVOS study): a secondary analysis from a randomised controlled trial.BMC Sports Sci Med Rehabil. 2020 Sep 5;12:54. doi: 10.1186/s13102-020-00204-w. eCollection 2020. BMC Sports Sci Med Rehabil. 2020. PMID: 32944251 Free PMC article.
-
The combined effect of Parathyroid hormone (1-34) and whole-body Vibration exercise in the treatment of postmenopausal OSteoporosis (PaVOS study): a randomized controlled trial.Osteoporos Int. 2019 Sep;30(9):1827-1836. doi: 10.1007/s00198-019-05029-z. Epub 2019 Jul 15. Osteoporos Int. 2019. PMID: 31309239 Free PMC article. Clinical Trial.
References
-
- World Health Organization. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO technical report series. Geneva: WHO; 1994. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- (1.31.72 Sagsnr.: 12/26914])/The Odense University Hospital Research Foundation
- (J.nr. 13/26005)/The Region of Southern Denmark Ph.D. Foundation
- (25-A1353)/The Research Foundation between Odense University Hospital and Rigshospitalet
- (15-177)/The A.P. Møller Foundation for the Advancement of Medical Science
- (15/36429)/OPEN- Odense Patient data Explorative Network
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

