Trophoblast differentiation, invasion and hormone secretion in a three-dimensional in vitro implantation model with rhesus monkey embryos
- PMID: 29548332
- PMCID: PMC5857108
- DOI: 10.1186/s12958-018-0340-3
Trophoblast differentiation, invasion and hormone secretion in a three-dimensional in vitro implantation model with rhesus monkey embryos
Abstract
Background: The initiation of primate embryo invasion into the endometrium and the formation of the placenta from trophoblasts, fetal mesenchyme, and vascular components are essential for the establishment of a successful pregnancy. The mechanisms which direct morphogenesis of the chorionic villi, and the interactions between trophectoderm-derived trophoblasts and the fetal mesenchyme to direct these processes during placentation are not well understood due to a dearth of systems to examine and manipulate real-time primate implantation. Here we describe an in vitro three-dimensional (3-D) model to study implantation which utilized IVF-generated rhesus monkey embryos cultured in a Matrigel explant system.
Methods: Blastocyst stage embryos were embedded in a 3-D microenvironment of a Matrigel carrier and co-cultured with a feeder layer of cells generating conditioned medium. Throughout the course of embryo co-culture embryo growth and secretions were monitored. Embedded embryos were then sectioned and stained for markers of trophoblast function and differentiation.
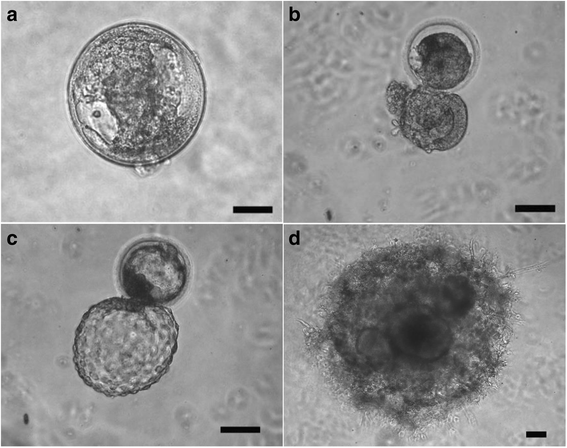
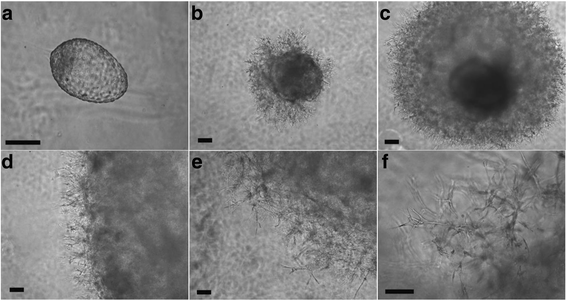
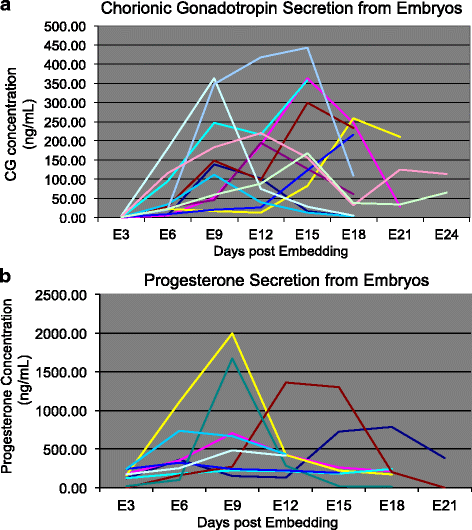
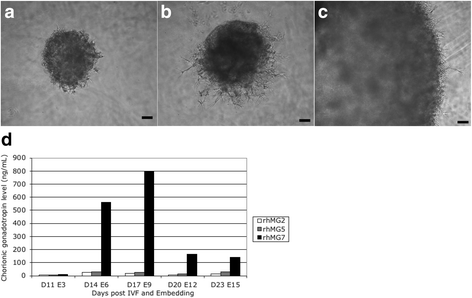
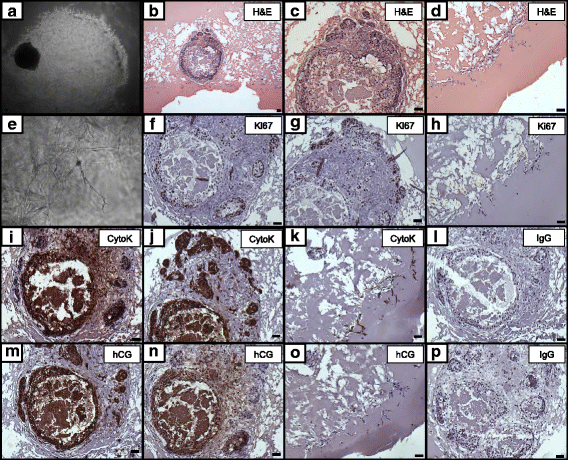
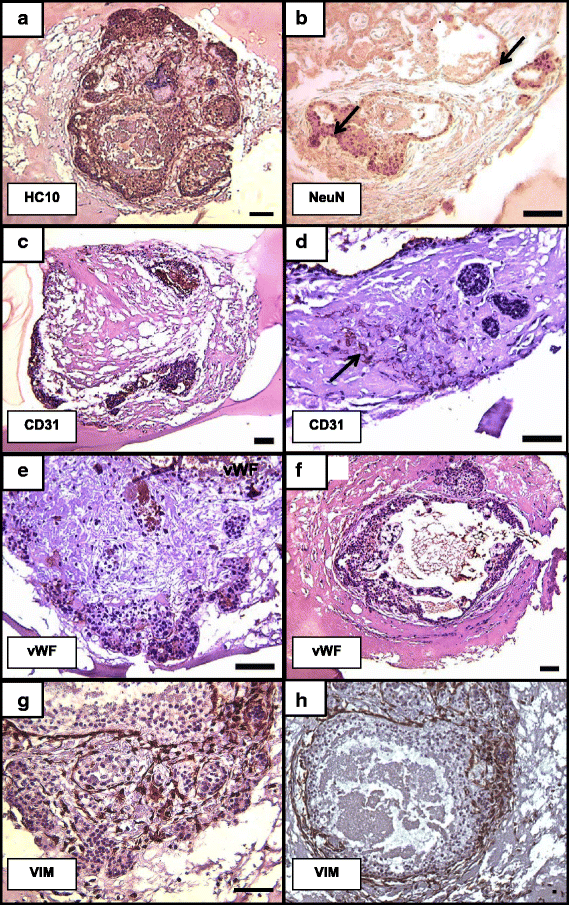
Results: Signs of implantation were observed including enlargement of the embryo mass, and invasion and proliferation of trophoblast outgrowths. Expression of chorionic gonadotropin defined by immunohistochemical staining, and secretion of chorionic gonadotropin and progesterone coincident with the appearance of trophoblast outgrowths, supported the conclusion that a trophoblast cell lineage formed from implanted embryos. Positive staining for selected markers including Ki67, MHC class I, NeuN, CD31, vonWillebrand Factor and Vimentin, suggest growth and differentiation of the embryo following embedding.
Conclusions: This 3-D in vitro system will facilitate further study of primate embryo biology, with potential to provide a platform for study of genes related to implantation defects and trophoblast differentiation.
Keywords: Embryo; Implantation; Non-human primate; Trophoblast.
Conflict of interest statement
Ethics approval
All animal procedures were performed in accordance with NIH Guide for the Care and Use of Laboratory Animals and under the approval of the University of Wisconsin Graduate School Animal Care and Use Committee.
Consent for publication
Not applicable
Competing interests
The authors declare they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Macrophages modulate the growth and differentiation of rhesus monkey embryonic trophoblasts.Am J Reprod Immunol. 2016 Nov;76(5):364-375. doi: 10.1111/aji.12564. Epub 2016 Sep 17. Am J Reprod Immunol. 2016. PMID: 27637575 Free PMC article.
-
[Studies on the mechanism of embryo implantation].Nihon Sanka Fujinka Gakkai Zasshi. 1996 Aug;48(8):591-603. Nihon Sanka Fujinka Gakkai Zasshi. 1996. PMID: 8808827 Japanese.
-
Trophectoderm differentiation to invasive syncytiotrophoblast is promoted by endometrial epithelial cells during human embryo implantation.Hum Reprod. 2022 Apr 1;37(4):777-792. doi: 10.1093/humrep/deac008. Hum Reprod. 2022. PMID: 35079788 Free PMC article.
-
[Chorionic gonadotropin as the key factor for embryo implantation].Ginekol Pol. 2008 Oct;79(10):692-6. Ginekol Pol. 2008. PMID: 19058524 Review. Polish.
-
Endometrial biology during trophoblast invasion.Front Biosci (Schol Ed). 2012 Jan 1;4(3):1151-71. doi: 10.2741/s323. Front Biosci (Schol Ed). 2012. PMID: 22202114 Review.
Cited by
-
CLDN3 expression and function in pregnancy-induced hypertension.Exp Ther Med. 2020 Oct;20(4):3798-3806. doi: 10.3892/etm.2020.9084. Epub 2020 Jul 31. Exp Ther Med. 2020. PMID: 32855729 Free PMC article.
-
Tuning Trophoblast Motility in a Gelatin Hydrogel via Soluble Cues from the Maternal-Fetal Interface.Tissue Eng Part A. 2021 Aug;27(15-16):1064-1073. doi: 10.1089/ten.tea.2020.0097. Epub 2020 Nov 20. Tissue Eng Part A. 2021. PMID: 33216701 Free PMC article.
-
Screening Candidate Genes Regulating Placental Development from Trophoblast Transcriptome at Early Pregnancy in Dazu Black Goats (Capra hircus).Animals (Basel). 2021 Jul 19;11(7):2132. doi: 10.3390/ani11072132. Animals (Basel). 2021. PMID: 34359260 Free PMC article.
-
Placenta-derived macaque trophoblast stem cells: differentiation to syncytiotrophoblasts and extravillous trophoblasts reveals phenotypic reprogramming.Sci Rep. 2020 Nov 5;10(1):19159. doi: 10.1038/s41598-020-76313-w. Sci Rep. 2020. PMID: 33154556 Free PMC article.
-
Embryotoxic impact of Zika virus in a rhesus macaque in vitro implantation model†.Biol Reprod. 2020 Apr 15;102(4):806-816. doi: 10.1093/biolre/ioz236. Biol Reprod. 2020. PMID: 31901091 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

