Catalytic modulation of human cytochromes P450 17A1 and P450 11B2 by phospholipid
- PMID: 29548669
- PMCID: PMC5992074
- DOI: 10.1016/j.jsbmb.2018.03.003
Catalytic modulation of human cytochromes P450 17A1 and P450 11B2 by phospholipid
Abstract
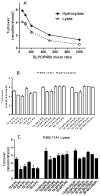
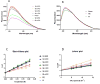
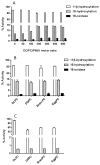
Unlike most of the drug-metabolizing cytochrome P450s, microsomal P450 17A1 and mitochondrial P450 11B2 catalyze sequential multi-step reactions in steroid biosynthesis. The membrane phospholipid composition might be one parameter that modulates the efficiency and processivity of specific pathways. Here we systematically examined the effects of physiologically relevant phospholipids on the catalysis of purified P450 17A1, P450 11B2, and P450 11B1 in reconstituted assay systems. Both dioleoylphosphatidylcholine (DOPC, 18:1) and dilauroylphosphatidylcholine (DLPC, 12:0) were found to be very efficient in reconstituting 17-hydroxylase and 1720-lyase reactions of P450 17A1. Phosphatidylethanolamine (PE) specifically enhanced 1720-lyase activity up to 2.4-fold in the presence of phosphatidylcholine. On the other hand, P450 11B2-catalyzed production of aldosterone from 11-deoxycorticosterone was very low and from 18-hydroxycorticosterone nil, implying low processivity. DOPC or cardiolipin, which is exclusively located in the inner mitochondrial membrane, maximized aldosterone yield. In sharp contrast, reconstitution of homologous P450 11B1 with DOPC significantly decreased corticosterone formation without affecting the synthesis of 18-hydroxycorticosterone. The intrinsic fluorescence of P450 17A1 and 11B2 increased in the presence of DOPC, DLPC and PE. Acrylamide quenching studies showed that PE decreased solvent accessibility for tryptophan in P450 17A1, as did 20:4 PC or 18:2 PC for P450 11B2. A moderately positive correlation between the proportion of high-spin substrate-bound species and catalytic activity was only observed in the presence of phosphatidylcholines with low-temperature phase transition. These results demonstrate the potential for phospholipids to regulate the activity of steroidogenic P450 activities and thereby steroid hormone biosynthetic pathways.
Keywords: P450 11B2; P450 17A1; Phospholipid.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Kinetics of Intermediate Release Enhances P450 11B2-Catalyzed Aldosterone Synthesis.Biochemistry. 2024 Apr 16;63(8):1026-1037. doi: 10.1021/acs.biochem.3c00725. Epub 2024 Apr 2. Biochemistry. 2024. PMID: 38564530 Free PMC article.
-
Human cytochrome P450 11B2 produces aldosterone by a processive mechanism due to the lactol form of the intermediate 18-hydroxycorticosterone.J Biol Chem. 2019 Aug 30;294(35):12975-12991. doi: 10.1074/jbc.RA119.009830. Epub 2019 Jul 11. J Biol Chem. 2019. PMID: 31296661 Free PMC article.
-
Molecular Recognition in Mitochondrial Cytochromes P450 That Catalyze the Terminal Steps of Corticosteroid Biosynthesis.Biochemistry. 2017 May 2;56(17):2282-2293. doi: 10.1021/acs.biochem.7b00034. Epub 2017 Apr 17. Biochemistry. 2017. PMID: 28355486 Free PMC article.
-
Role of cytochrome b5 in the modulation of the enzymatic activities of cytochrome P450 17α-hydroxylase/17,20-lyase (P450 17A1).J Steroid Biochem Mol Biol. 2017 Jun;170:2-18. doi: 10.1016/j.jsbmb.2016.02.033. Epub 2016 Mar 11. J Steroid Biochem Mol Biol. 2017. PMID: 26976652 Review.
-
Updates on Mechanisms of Cytochrome P450 Catalysis of Complex Steroid Oxidations.Int J Mol Sci. 2024 Aug 20;25(16):9020. doi: 10.3390/ijms25169020. Int J Mol Sci. 2024. PMID: 39201706 Free PMC article. Review.
Cited by
-
Role of tryptophan-metabolizing microbiota in mice diarrhea caused by Folium sennae extracts.BMC Microbiol. 2020 Jun 29;20(1):185. doi: 10.1186/s12866-020-01864-x. BMC Microbiol. 2020. PMID: 32600333 Free PMC article.
-
Selectivity of osilodrostat as an inhibitor of human steroidogenic cytochromes P450.J Steroid Biochem Mol Biol. 2023 Jul;231:106316. doi: 10.1016/j.jsbmb.2023.106316. Epub 2023 Apr 23. J Steroid Biochem Mol Biol. 2023. PMID: 37098354 Free PMC article.
-
Kinetics of Intermediate Release Enhances P450 11B2-Catalyzed Aldosterone Synthesis.Biochemistry. 2024 Apr 16;63(8):1026-1037. doi: 10.1021/acs.biochem.3c00725. Epub 2024 Apr 2. Biochemistry. 2024. PMID: 38564530 Free PMC article.
-
Human cytochrome P450 11B2 produces aldosterone by a processive mechanism due to the lactol form of the intermediate 18-hydroxycorticosterone.J Biol Chem. 2019 Aug 30;294(35):12975-12991. doi: 10.1074/jbc.RA119.009830. Epub 2019 Jul 11. J Biol Chem. 2019. PMID: 31296661 Free PMC article.
-
Congenital Adrenal Hyperplasia-Current Insights in Pathophysiology, Diagnostics, and Management.Endocr Rev. 2022 Jan 12;43(1):91-159. doi: 10.1210/endrev/bnab016. Endocr Rev. 2022. PMID: 33961029 Free PMC article. Review.
References
-
- Auchus RJ, Lee TC, Miller WL. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem. 1998;273:3158–3165. - PubMed
-
- Rainey WE. Adrenal zonation: clues from 11β-hydroxylase and aldosterone synthase. Mol Cell Endocrinol. 1999;151:151–160. - PubMed
-
- Bureik M, Lisurek M, Bernhardt R. The human steroid hydroxylases CYP1B1 and CYP11B2. Biol Chem. 2002;383:1537–1551. - PubMed
-
- Omura T. Structural diversity of cytochrome P450 enzyme system. J Biochem. 2010;147:297–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

