Activation of adenosine A2A or A2B receptors causes hypothermia in mice
- PMID: 29548686
- PMCID: PMC6067974
- DOI: 10.1016/j.neuropharm.2018.02.035
Activation of adenosine A2A or A2B receptors causes hypothermia in mice
Abstract
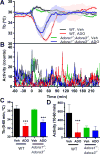
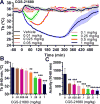
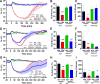
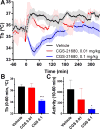
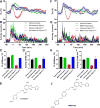
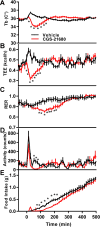
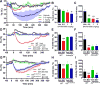
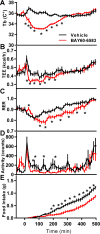
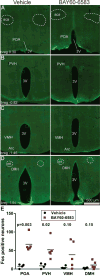
Extracellular adenosine is a danger/injury signal that initiates protective physiology, such as hypothermia. Adenosine has been shown to trigger hypothermia via agonism at A1 and A3 adenosine receptors (A1AR, A3AR). Here, we find that adenosine continues to elicit hypothermia in mice null for A1AR and A3AR and investigated the effect of agonism at A2AAR or A2BAR. The poorly brain penetrant A2AAR agonists CGS-21680 and PSB-0777 caused hypothermia, which was not seen in mice lacking A2AAR. MRS7352, a likely non-brain penetrant A2AAR antagonist, inhibited PSB-0777 hypothermia. While vasodilation is probably a contributory mechanism, A2AAR agonism also caused hypometabolism, indicating that vasodilation is not the sole mechanism. The A2BAR agonist BAY60-6583 elicited hypothermia, which was lost in mice null for A2BAR. Low intracerebroventricular doses of BAY60-6583 also caused hypothermia, indicating a brain site of action, with neuronal activation in the preoptic area and paraventricular nucleus of the hypothalamus. Thus, agonism at any one of the canonical adenosine receptors, A1AR, A2AAR, A2BAR, or A3AR, can cause hypothermia. This four-fold redundancy in adenosine-mediated initiation of hypothermia may reflect the centrality of hypothermia as a protective response.
Keywords: A(2A)AR; A(2B)AR; Adenosine; Hypothermia; Paraventricular hypothalamus; Preoptic area.
Published by Elsevier Ltd.
Figures

References
-
- Al Jaroudi W, Iskandrian AE. Regadenoson: a new myocardial stress agent. J Am Coll Cardiol. 2009;54:1123–1130. - PubMed
-
- Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD. Effects of hypothermia for perinatal asphyxia on childhood outcomes. The New England journal of medicine. 2014;371:140–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

