CaBP1 regulates Cav1 L-type Ca2+ channels and their coupling to neurite growth and gene transcription in mouse spiral ganglion neurons
- PMID: 29548764
- PMCID: PMC6013052
- DOI: 10.1016/j.mcn.2018.03.005
CaBP1 regulates Cav1 L-type Ca2+ channels and their coupling to neurite growth and gene transcription in mouse spiral ganglion neurons
Abstract
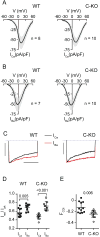
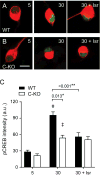
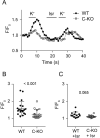
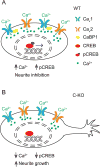
CaBP1 is a Ca2+ binding protein that is widely expressed in neurons in the brain, retina, and cochlea. In heterologous expression systems, CaBP1 interacts with and regulates voltage-gated Cav Ca2+ channels but whether this is the case in neurons is unknown. Here, we investigated the cellular functions of CaBP1 in cochlear spiral ganglion neurons (SGNs), which express high levels of CaBP1. Consistent with the role of CaBP1 as a suppressor of Ca2+-dependent inactivation (CDI) of Cav1 (L-type) channels, Cav1 currents underwent greater CDI in SGNs from mice lacking CaBP1 (C-KO) than in wild-type (WT) SGNs. The coupling of Cav1 channels to downstream signaling pathways was also disrupted in C-KO SGNs. Activity-dependent repression of neurite growth was significantly blunted and unresponsive to Cav1 antagonists in C-KO SGNs in contrast to WT SGNs. Moreover, Cav1-mediated Ca2+ signals and phosphorylation of cAMP-response element binding protein were reduced in C-KO SGNs compared to WT SGNs. Our findings establish a role for CaBP1 as an essential regulator of Cav1 channels in SGNs and their coupling to downstream pathways controlling activity-dependent transcription and neurite growth.
Keywords: Auditory; Ca(2+) channels; Neurite growth; Spiral ganglion.
Copyright © 2018. Published by Elsevier Inc.
Figures





Similar articles
-
Genetic, cellular, and functional evidence for Ca2+ inflow through Cav1.2 and Cav1.3 channels in murine spiral ganglion neurons.J Neurosci. 2014 May 21;34(21):7383-93. doi: 10.1523/JNEUROSCI.5416-13.2014. J Neurosci. 2014. PMID: 24849370 Free PMC article.
-
Caldendrin, a neuron-specific modulator of Cav/1.2 (L-type) Ca2+ channels.J Biol Chem. 2007 Mar 16;282(11):8464-73. doi: 10.1074/jbc.M611384200. Epub 2007 Jan 15. J Biol Chem. 2007. PMID: 17224447
-
Functions of CaBP1 and CaBP2 in the peripheral auditory system.Hear Res. 2018 Jul;364:48-58. doi: 10.1016/j.heares.2018.04.001. Epub 2018 Apr 9. Hear Res. 2018. PMID: 29661613 Free PMC article.
-
L-Type Ca2+ Channel Regulation by Calmodulin and CaBP1.Biomolecules. 2021 Dec 2;11(12):1811. doi: 10.3390/biom11121811. Biomolecules. 2021. PMID: 34944455 Free PMC article. Review.
-
Decalmodulation of Cav1 channels by CaBPs.Channels (Austin). 2016;10(1):33-7. doi: 10.1080/19336950.2015.1051273. Epub 2015 Jul 8. Channels (Austin). 2016. PMID: 26155893 Free PMC article. Review.
Cited by
-
Caldendrin represses neurite regeneration and growth in dorsal root ganglion neurons.Sci Rep. 2023 Feb 14;13(1):2608. doi: 10.1038/s41598-023-29622-9. Sci Rep. 2023. PMID: 36788334 Free PMC article.
-
NeuriteNet: A convolutional neural network for assessing morphological parameters of neurite growth.J Neurosci Methods. 2021 Nov 1;363:109349. doi: 10.1016/j.jneumeth.2021.109349. Epub 2021 Sep 2. J Neurosci Methods. 2021. PMID: 34480956 Free PMC article.
-
Caldendrin Is a Repressor of PIEZO2 Channels and Touch Sensation in Mice.J Neurosci. 2024 Mar 6;44(10):e1402232023. doi: 10.1523/JNEUROSCI.1402-23.2023. J Neurosci. 2024. PMID: 38262725 Free PMC article.
-
Calcium Sensors in Neuronal Function and Dysfunction.Cold Spring Harb Perspect Biol. 2019 May 1;11(5):a035154. doi: 10.1101/cshperspect.a035154. Cold Spring Harb Perspect Biol. 2019. PMID: 30833454 Free PMC article. Review.
-
Structure-Function Diversity of Calcium-Binding Proteins (CaBPs): Key Roles in Cell Signalling and Disease.Cells. 2025 Jan 21;14(3):152. doi: 10.3390/cells14030152. Cells. 2025. PMID: 39936944 Free PMC article. Review.
References
-
- Audesirk G, Audesirk T, Ferguson C, Lomme M, Shugarts D, Rosack J, Caracciolo P, Gisi T, Nichols P. L-type calcium channels may regulate neurite initiation in cultured chick embryo brain neurons and N1E-115 neuroblastoma cells. Brain Res Dev Brain Res. 1990;55:109–120. - PubMed
-
- Bengtson CP, Bading H. Nuclear calcium signaling. Adv Exp Med Biol. 2012;970:377–405. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

