Regulation of protein homeostasis by unconventional protein secretion in mammalian cells
- PMID: 29549062
- PMCID: PMC6151168
- DOI: 10.1016/j.semcdb.2018.03.006
Regulation of protein homeostasis by unconventional protein secretion in mammalian cells
Abstract
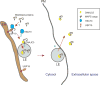
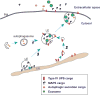
Secretion of proteins lacking leader sequence was deemed rare and unconventional, only accountable for the export of a limited number of clients by mechanisms that are poorly defined. However, recent studies have shown that many leaderless proteins misfolded in the cytoplasm can be selectively exported to extracellular milieu via an unconventional secretory path termed Misfolding-Associated Protein Secretion (MAPS). This process uses the surface of the endoplasmic reticulum (ER) as a platform to enrich abnormally folded polypeptides, and then transport them into the lumen of ER-associated late endosomes for subsequent secretion. Elimination of misfolded proteins via MAPS appears to serve a role in protein homeostasis maintenance, particularly for stressed cells bearing an excess of protein quality control (PQC) burden.
Keywords: DNAJC5/CSP; HSC70; Misfolding-associated protein secretion (MAPS); USP19; Unconventional protein secretion; cell-to-cell transmission; neurodegenerative diseases; proteasome; protein quality control; α-Synuclein.
Published by Elsevier Ltd.
Figures

Similar articles
-
Unconventional secretion of misfolded proteins promotes adaptation to proteasome dysfunction in mammalian cells.Nat Cell Biol. 2016 Jul;18(7):765-76. doi: 10.1038/ncb3372. Epub 2016 Jun 13. Nat Cell Biol. 2016. PMID: 27295555 Free PMC article.
-
DNAJC5 facilitates USP19-dependent unconventional secretion of misfolded cytosolic proteins.Cell Discov. 2018 Mar 6;4:11. doi: 10.1038/s41421-018-0012-7. eCollection 2018. Cell Discov. 2018. PMID: 29531792 Free PMC article.
-
The Roles of Endo-Lysosomes in Unconventional Protein Secretion.Cells. 2018 Nov 3;7(11):198. doi: 10.3390/cells7110198. Cells. 2018. PMID: 30400277 Free PMC article. Review.
-
Enhanced secretion of the amyotrophic lateral sclerosis ALS-associated misfolded TDP-43 mediated by the ER-ubiquitin specific peptidase USP19.Cell Mol Life Sci. 2025 Feb 13;82(1):76. doi: 10.1007/s00018-025-05589-w. Cell Mol Life Sci. 2025. PMID: 39948244 Free PMC article.
-
Proteostasis regulation at the endoplasmic reticulum: a new perturbation site for targeted cancer therapy.Cell Res. 2011 Jun;21(6):867-83. doi: 10.1038/cr.2011.75. Epub 2011 May 3. Cell Res. 2011. PMID: 21537343 Free PMC article. Review.
Cited by
-
Cooperation of cell adhesion and autophagy in the brain: Functional roles in development and neurodegenerative disease.Matrix Biol Plus. 2021 Oct 23;12:100089. doi: 10.1016/j.mbplus.2021.100089. eCollection 2021 Dec. Matrix Biol Plus. 2021. PMID: 34786551 Free PMC article. Review.
-
Cell Responses to Extracellular α-Synuclein.Molecules. 2019 Jan 15;24(2):305. doi: 10.3390/molecules24020305. Molecules. 2019. PMID: 30650656 Free PMC article. Review.
-
Bortezomib Is Toxic but Induces Neurogenesis and Inhibits TUBB3 Degradation in Rat Neural Stem Cells.Biomol Ther (Seoul). 2024 Jan 1;32(1):65-76. doi: 10.4062/biomolther.2023.134. Epub 2023 Dec 11. Biomol Ther (Seoul). 2024. PMID: 38072501 Free PMC article.
-
The bacterial toxin ExoU requires a host trafficking chaperone for transportation and to induce necrosis.Nat Commun. 2021 Jun 29;12(1):4024. doi: 10.1038/s41467-021-24337-9. Nat Commun. 2021. PMID: 34188051 Free PMC article.
-
Filamentous recombinant human Tau activates primary astrocytes via an integrin receptor complex.Nat Commun. 2021 Jan 4;12(1):95. doi: 10.1038/s41467-020-20322-w. Nat Commun. 2021. PMID: 33398028 Free PMC article.
References
-
- Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2011;40(2):238–52. - PubMed
-
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292(5521):1552–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

