Regulation of protein homeostasis by unconventional protein secretion in mammalian cells
- PMID: 29549062
- PMCID: PMC6151168
- DOI: 10.1016/j.semcdb.2018.03.006
Regulation of protein homeostasis by unconventional protein secretion in mammalian cells
Abstract
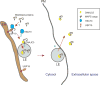
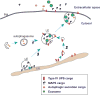
Secretion of proteins lacking leader sequence was deemed rare and unconventional, only accountable for the export of a limited number of clients by mechanisms that are poorly defined. However, recent studies have shown that many leaderless proteins misfolded in the cytoplasm can be selectively exported to extracellular milieu via an unconventional secretory path termed Misfolding-Associated Protein Secretion (MAPS). This process uses the surface of the endoplasmic reticulum (ER) as a platform to enrich abnormally folded polypeptides, and then transport them into the lumen of ER-associated late endosomes for subsequent secretion. Elimination of misfolded proteins via MAPS appears to serve a role in protein homeostasis maintenance, particularly for stressed cells bearing an excess of protein quality control (PQC) burden.
Keywords: DNAJC5/CSP; HSC70; Misfolding-associated protein secretion (MAPS); USP19; Unconventional protein secretion; cell-to-cell transmission; neurodegenerative diseases; proteasome; protein quality control; α-Synuclein.
Published by Elsevier Ltd.
Figures

References
-
- Buchberger A, Bukau B, Sommer T. Protein quality control in the cytosol and the endoplasmic reticulum: brothers in arms. Mol Cell. 2011;40(2):238–52. - PubMed
-
- Bence NF, Sampat RM, Kopito RR. Impairment of the ubiquitin-proteasome system by protein aggregation. Science. 2001;292(5521):1552–5. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

