A Single-Nucleotide Insertion in a Drug Transporter Gene Induces a Thermotolerance Phenotype in Gluconobacter frateurii by Increasing the NADPH/NADP+ Ratio via Metabolic Change
- PMID: 29549098
- PMCID: PMC5930370
- DOI: 10.1128/AEM.00354-18
A Single-Nucleotide Insertion in a Drug Transporter Gene Induces a Thermotolerance Phenotype in Gluconobacter frateurii by Increasing the NADPH/NADP+ Ratio via Metabolic Change
Abstract
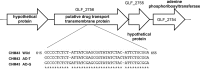
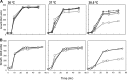
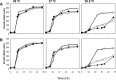
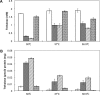
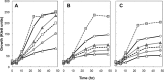
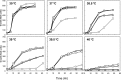
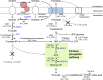
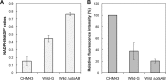
Thermotolerant microorganisms are beneficial to the fermentation industry because they reduce the need for cooling and offer other operational advantages. Previously, we obtained a thermally adapted Gluconobacter frateurii strain by experimental evolution. In the present study, we found only a single G insertion in the adapted strain, which causes a frameshift in a gene encoding a putative drug transporter. A mutant derivative strain with the single G insertion in the transporter gene (Wild-G) was constructed from the wild-type strain and showed increased thermotolerance. We found that the thermotolerant strains accumulated substantial intracellular trehalose and manifested a defect in sorbose assimilation, suggesting that the transporter is partly involved in trehalose efflux and sorbose uptake and that the defect in the transporter can improve thermotolerance. The ΔotsAB strain, constructed by elimination of the trehalose synthesis gene in the wild type, showed no trehalose production but, unexpectedly, much better growth than the adapted strain at high temperatures. The ΔotsAB mutant produced more acetate as the final metabolite than the wild-type strain did. We hypothesized that trehalose does not contribute to thermotolerance directly; rather, a metabolic change including increased carbon flux to the pentose phosphate pathway may be the key factor. The NADPH/NADP+ ratio was higher in strain Wild-G, and much higher in the ΔotsAB strain, than in the wild-type strain. Levels of reactive oxygen species (ROS) were lower in the thermotolerant strains. We propose that the defect of the transporter causes the metabolic flux to generate more NADPH, which may enhance thermotolerance in G. frateuriiIMPORTANCE The biorefinery industry has to ensure that microorganisms are robust and retain their viability and function at high temperatures. Here we show that Gluconobacterfrateurii, an industrially important member of the acetic acid bacteria, exhibited enhanced thermotolerance through the reduction of trehalose excretion after thermal adaptation. Although intracellular trehalose may play a key role in thermotolerance, the molecular mechanisms of action of trehalose in thermotolerance are a matter of debate. Our mutated strain that was defective in trehalose synthase genes, producing no trehalose but a larger amount of acetic acid as the end metabolite instead, unexpectedly showed higher thermotolerance than the wild type. Our adapted and mutated thermotolerant strains showed increased NADPH/NADP+ ratios and reductions in ROS levels. We concluded that in G. frateurii, trehalose does not contribute to thermotolerance directly; rather, the metabolic change increases the NADPH/NADP+ ratio to enhance thermotolerance.
Keywords: Gluconobacter; NADPH/NADP+ ratio; acetic acid bacteria; drug transporter; reactive oxygen species; thermal adaptation; thermotolerance; trehalose.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
High-temperature sorbose fermentation with thermotolerant Gluconobacter frateurii CHM43 and its mutant strain adapted to higher temperature.Appl Microbiol Biotechnol. 2012 Sep;95(6):1531-40. doi: 10.1007/s00253-012-4005-4. Epub 2012 Mar 22. Appl Microbiol Biotechnol. 2012. PMID: 22434571
-
L-sorbose reductase and its transcriptional regulator involved in L-sorbose utilization of Gluconobacter frateurii.J Bacteriol. 2007 Jul;189(13):4800-8. doi: 10.1128/JB.01895-06. Epub 2007 Apr 27. J Bacteriol. 2007. PMID: 17468249 Free PMC article.
-
Isolation and characterization of thermotolerant Gluconobacter strains catalyzing oxidative fermentation at higher temperatures.Biosci Biotechnol Biochem. 2000 Nov;64(11):2306-15. doi: 10.1271/bbb.64.2306. Biosci Biotechnol Biochem. 2000. PMID: 11193396
-
New developments in oxidative fermentation.Appl Microbiol Biotechnol. 2003 Feb;60(6):643-53. doi: 10.1007/s00253-002-1155-9. Epub 2002 Dec 18. Appl Microbiol Biotechnol. 2003. PMID: 12664142 Review.
-
Genomic analyses of thermotolerant microorganisms used for high-temperature fermentations.Biosci Biotechnol Biochem. 2016;80(4):655-68. doi: 10.1080/09168451.2015.1104235. Epub 2015 Nov 13. Biosci Biotechnol Biochem. 2016. PMID: 26566045 Review.
Cited by
-
Trehalose alleviates high-temperature stress in Pleurotus ostreatus by affecting central carbon metabolism.Microb Cell Fact. 2021 Apr 7;20(1):82. doi: 10.1186/s12934-021-01572-9. Microb Cell Fact. 2021. PMID: 33827585 Free PMC article.
-
On the way toward regulatable expression systems in acetic acid bacteria: target gene expression and use cases.Appl Microbiol Biotechnol. 2021 May;105(9):3423-3456. doi: 10.1007/s00253-021-11269-z. Epub 2021 Apr 15. Appl Microbiol Biotechnol. 2021. PMID: 33856535 Free PMC article. Review.
-
Mutations in degP and spoT Genes Mediate Response to Fermentation Stress in Thermally Adapted Strains of Acetic Acid Bacterium Komagataeibacter medellinensis NBRC 3288.Front Microbiol. 2022 May 12;13:802010. doi: 10.3389/fmicb.2022.802010. eCollection 2022. Front Microbiol. 2022. PMID: 35633714 Free PMC article.
-
Heat stress in macrofungi: effects and response mechanisms.Appl Microbiol Biotechnol. 2021 Oct;105(20):7567-7576. doi: 10.1007/s00253-021-11574-7. Epub 2021 Sep 18. Appl Microbiol Biotechnol. 2021. PMID: 34536103 Review.
-
Two NADPH-dependent 2-ketogluconate reductases involved in 2-ketogluconate assimilation in Gluconobacter sp. strain CHM43.Appl Environ Microbiol. 2025 Feb 19;91(2):e0250124. doi: 10.1128/aem.02501-24. Epub 2025 Jan 29. Appl Environ Microbiol. 2025. PMID: 39878490 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

