Adipokinetic hormone signaling determines dietary fatty acid preference through maintenance of hemolymph fatty acid composition in the cricket Gryllus bimaculatus
- PMID: 29549314
- PMCID: PMC5856772
- DOI: 10.1038/s41598-018-22987-2
Adipokinetic hormone signaling determines dietary fatty acid preference through maintenance of hemolymph fatty acid composition in the cricket Gryllus bimaculatus
Abstract
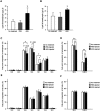
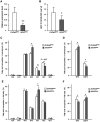
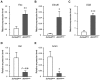
Adipokinetic hormone (AKH), an analog of mammalian glucagon, functions in supplying the required energy by releasing lipids and carbohydrates from the fat body into the hemolymph. Our previous study showed that AKH receptor (AKHR) knockdown in the two-spotted cricket Gryllus bimaculatus decreased hemolymph lipid levels and increased its feeding frequency. To reveal underlying mechanisms by which AKH signaling modulates lipid homeostasis, we analyzed the fatty acid composition as the lipid structure in the crickets. AKH administration significantly increased the proportion of unsaturated fatty acids (USFAs) to total fatty acids with decrease of the saturated fatty acids (SFAs) in hemolymph, while these proportions were inversely changed in RNA interference-mediated AKHR-knockdowned (AKHRRNAi) crickets. Interestingly, knockdown of hormone-sensitive lipase (Hsl) by RNAi (HslRNAi) affected the proportion of USFAs and SFAs in a similar manner to that observed in AKHRRNAi crickets. AKH administration in HslRNAi crickets did not change hemolymph fatty acid composition, indicating that AKH signaling critically altered fatty acid composition in the hemolymph through Hsl. In addition, a choice assay revealed that AKHRRNAi significantly increases the preference of USFAs. These data indicate that hemolymph lipid level and composition were modulated by AKH signaling with a complementary feeding behavior toward USFAs.
Conflict of interest statement
The authors declare no competing interests.
Figures
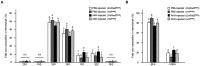

Similar articles
-
Imbalanced Hemolymph Lipid Levels Affect Feeding Motivation in the Two-Spotted Cricket, Gryllus bimaculatus.PLoS One. 2016 May 4;11(5):e0154841. doi: 10.1371/journal.pone.0154841. eCollection 2016. PLoS One. 2016. PMID: 27144650 Free PMC article.
-
Adipokinetic hormone inhibits the formation of energy stores and egg production in the cricket Gryllus bimaculatus.Comp Biochem Physiol B Biochem Mol Biol. 2003 Oct;136(2):197-206. doi: 10.1016/s1096-4959(03)00227-6. Comp Biochem Physiol B Biochem Mol Biol. 2003. PMID: 14529746
-
Effects of adipokinetic hormone and its related peptide on maintaining hemolymph carbohydrate and lipid levels in the two-spotted cricket, Gryllus bimaculatus.Biosci Biotechnol Biochem. 2018 Feb;82(2):274-284. doi: 10.1080/09168451.2017.1422106. Epub 2018 Jan 12. Biosci Biotechnol Biochem. 2018. PMID: 29325488
-
The Intrinsic Nutrient Sensing Adipokinetic Hormone Producing Cells Function in Modulation of Metabolism, Activity, and Stress.Int J Mol Sci. 2021 Jul 13;22(14):7515. doi: 10.3390/ijms22147515. Int J Mol Sci. 2021. PMID: 34299134 Free PMC article. Review.
-
New insights into adipokinetic hormone signaling.Mol Cell Endocrinol. 1998 Jun 25;141(1-2):7-12. doi: 10.1016/s0303-7207(98)00079-3. Mol Cell Endocrinol. 1998. PMID: 9723879 Review.
Cited by
-
CCHamide-2 Signaling Regulates Food Intake and Metabolism in Gryllus bimaculatus.Insects. 2022 Mar 25;13(4):324. doi: 10.3390/insects13040324. Insects. 2022. PMID: 35447766 Free PMC article.
-
Establishment of an induced memory response in Pseudomonas aeruginosa during infection of a eukaryotic host.ISME J. 2019 Aug;13(8):2018-2030. doi: 10.1038/s41396-019-0412-1. Epub 2019 Apr 5. ISME J. 2019. PMID: 30952997 Free PMC article.
-
A Novel Head Capsule Labial Gland Lobe in the Black Field Cricket (Orthoptera: Gryllidae).J Insect Sci. 2020 Jul 1;20(4):8. doi: 10.1093/jisesa/ieaa068. J Insect Sci. 2020. PMID: 32697826 Free PMC article.
-
Best Practices for Comprehensive Annotation of Neuropeptides of Gryllus bimaculatus.Insects. 2023 Jan 25;14(2):121. doi: 10.3390/insects14020121. Insects. 2023. PMID: 36835690 Free PMC article.
-
Identification and Characterization of 24-Dehydrocholesterol Reductase (DHCR24) in the Two-Spotted Cricket, Gryllus bimaculatus.Insects. 2021 Sep 1;12(9):782. doi: 10.3390/insects12090782. Insects. 2021. PMID: 34564222 Free PMC article.
References
-
- Mayer RJ, Candy DJ. Control of haemolymph lipid concentration during locust flight: An adipokinetic hormone from the corpora cardiaca. J. Insect Physiol. 1969;15:611–620. doi: 10.1016/0022-1910(69)90259-5. - DOI
-
- Beenakkers AMT. Transport of fatty acids in Locusta migratoria during sustained flight. J. Insect Physiol. 1965;11:879–888. doi: 10.1016/0022-1910(65)90190-3. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

