Scalable total synthesis and comprehensive structure-activity relationship studies of the phytotoxin coronatine
- PMID: 29549326
- PMCID: PMC5856746
- DOI: 10.1038/s41467-018-03443-1
Scalable total synthesis and comprehensive structure-activity relationship studies of the phytotoxin coronatine
Abstract
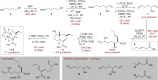
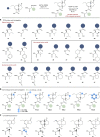
Natural phytotoxins are valuable starting points for agrochemical design. Acting as a jasmonate agonist, coronatine represents an attractive herbicidal lead with novel mode of action, and has been an important synthetic target for agrochemical development. However, both restricted access to quantities of coronatine and a lack of a suitably scalable and flexible synthetic approach to its constituent natural product components, coronafacic and coronamic acids, has frustrated development of this target. Here, we report gram-scale production of coronafacic acid that allows a comprehensive structure-activity relationship study of this target. Biological assessment of a >120 member library combined with computational studies have revealed the key determinants of potency, rationalising hypotheses held for decades, and allowing future rational design of new herbicidal leads based on this template.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

