A proteomic insight into vitellogenesis during tick ovary maturation
- PMID: 29549327
- PMCID: PMC5856802
- DOI: 10.1038/s41598-018-23090-2
A proteomic insight into vitellogenesis during tick ovary maturation
Abstract
Ticks are arthropod ectoparasites of importance for public and veterinary health. The understanding of tick oogenesis and embryogenesis could contribute to the development of novel control methods. However, to date, studies on the temporal dynamics of proteins during ovary development were not reported. In the present study we followed protein profile during ovary maturation. Proteomic analysis of ovary extracts was performed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using shotgun strategy, in addition to dimethyl labelling-based protein quantification. A total of 3,756 proteins were identified, which were functionally annotated into 30 categories. Circa 80% of the annotated proteins belong to categories related to basal metabolism, such as protein synthesis and modification machineries, nuclear regulation, cytoskeleton, proteasome machinery, transcriptional machinery, energetic metabolism, extracellular matrix/cell adhesion, immunity, oxidation/detoxification metabolism, signal transduction, and storage. The abundance of selected proteins involved in yolk uptake and degradation, as well as vitellin accumulation during ovary maturation, was assessed using dimethyl-labelling quantification. In conclusion, proteins identified in this study provide a framework for future studies to elucidate tick development and validate candidate targets for novel control methods.
Conflict of interest statement
The authors declare no competing interests.
Figures




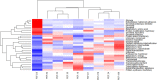


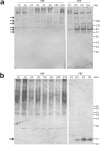

References
-
- Oliver JH. Biology and systematics of ticks (Acari:Ixodida) Annu. Rev. Ecol. Syst. 1989;20:397–430. doi: 10.1146/annurev.es.20.110189.002145. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

