Impact of atypical mitochondrial cyclic-AMP level in nephropathic cystinosis
- PMID: 29549422
- PMCID: PMC11105431
- DOI: 10.1007/s00018-018-2800-5
Impact of atypical mitochondrial cyclic-AMP level in nephropathic cystinosis
Abstract
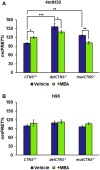
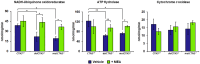
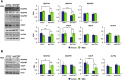
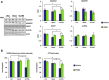
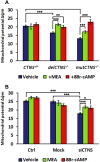
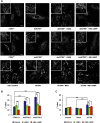
Nephropathic cystinosis (NC) is a rare disease caused by mutations in the CTNS gene encoding for cystinosin, a lysosomal transmembrane cystine/H+ symporter, which promotes the efflux of cystine from lysosomes to cytosol. NC is the most frequent cause of Fanconi syndrome (FS) in young children, the molecular basis of which is not well established. Proximal tubular cells have very high metabolic rate due to the active transport of many solutes. Not surprisingly, mitochondrial disorders are often characterized by FS. A similar mechanism may also apply to NC. Because cAMP has regulatory properties on mitochondrial function, we have analyzed cAMP levels and mitochondrial targets in CTNS-/- conditionally immortalized proximal tubular epithelial cells (ciPTEC) carrying the classical homozygous 57-kb deletion (delCTNS-/-) or with compound heterozygous loss-of-function mutations (mutCTNS-/-). Compared to wild-type cells, cystinotic cells had significantly lower mitochondrial cAMP levels (delCTNS-/- ciPTEC by 56% ± 10.5, P < 0.0001; mutCTNS-/- by 26% ± 4.3, P < 0.001), complex I and V activities, mitochondrial membrane potential, and SIRT3 protein levels, which were associated with increased mitochondrial fragmentation. Reduction of complex I and V activities was associated with lower expression of part of their subunits. Treatment with the non-hydrolysable cAMP analog 8-Br-cAMP restored mitochondrial potential and corrected mitochondria morphology. Treatment with cysteamine, which reduces the intra-lysosomal cystine, was able to restore mitochondrial cAMP levels, as well as most other abnormal mitochondrial findings. These observations were validated in CTNS-silenced HK-2 cells, indicating a pivotal role of mitochondrial cAMP in the proximal tubular dysfunction observed in NC.
Keywords: Cyclic-AMP; Cystinosis; Fanconi syndrome; Lysosomal storage disease; Mitochondria; SIRT3.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Bartsocas CS, Bernstein J, Orloff S, Chandra R, Schulman JD. A familial syndrome of growth retardation, severe Fanconi-type renal disease and glomerular changes–a new entity? Int J Pediatr Nephrol. 1986;7:101–106. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

