Deciphering interactions of ionic liquids with biomembrane
- PMID: 29549587
- PMCID: PMC5988625
- DOI: 10.1007/s12551-018-0410-y
Deciphering interactions of ionic liquids with biomembrane
Abstract
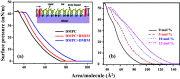
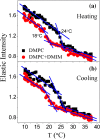
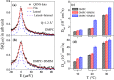
Ionic liquids (ILs) are a special class of low-temperature (typically < 100 °C) molten salts, which have huge upsurge interest in the field of chemical synthesis, catalysis, electrochemistry, pharmacology, and biotechnology, mainly due to their highly tunable nature and exceptional properties. However, practical uses of ILs are restricted mainly due to their adverse actions on organisms. Understanding interactions of ILs with biomembrane is prerequisite to assimilate the actions of these ionic compounds on the organism. Here, we review different biophysical methods to characterize interactions between ILs and phospholipid membrane, a model biomembrane. All these studies indicate that ILs interact profoundly with the lipid bilayer and modulate the structure, microscopic dynamics, and phase behavior of the membrane, which could be the fundamental cause of the observed toxicity of ILs. Effects of ILs on the membrane are found to be strongly dependent on the lipophilicity of the IL and are found to increase with the alkyl chain length of IL. This can be correlated with the observed higher toxicity of IL with the longer alkyl chain length. These informations would be useful to tune the toxicity of IL which is required in designing environment-friendly nontoxic solvents of the so-called green chemistry for various practical applications.
Keywords: Ionic liquids; Neutron scattering; Phase behavior; Phospholipid membrane; Structure and dynamics; Toxicity.
Conflict of interest statement
V. K. Sharma declares that he has no conflict of interest. R. Mukhopadhyay declares that he has no conflict of interest.
Figures





References
-
- Anastas PT, Zimmerman JB. Design through the 12 principles of green engineering. Environ Sci Technol. 2003;37(5):94A–101A. - PubMed
-
- Armstrong CL (2013) Diffusion and domains: membrane structure and dynamics studied by neutron scattering. Doctoral Thesis, McMaster University, Canada
-
- Bee M. Quasielastic neutron scattering: principles and applications in solid state chemistry, biology and materials science. Bristol: Adam Hilger; 1988.
-
- Benedetto A, Heinrich F, Gonzalez MA, Fragneto G, Watkins E, Ballone P. Structure and stability of phospholipid bilayers hydrated by a room-temperature ionic liquid/water solution: a neutron reflectometry study. J Phys Chem B. 2014;118:12192–12206. - PubMed
-
- Benedetto A, Bingham RJ, Ballone P. Structure and dynamics of POPC bilayers in water solutions of room temperature ionic liquids. J Chem Phys. 2015;142:124706. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

