Keratinocyte Growth Factor (KGF) Modulates Epidermal Progenitor Cell Kinetics through Activation of p63 in Middle Ear Cholesteatoma
- PMID: 29549594
- PMCID: PMC5962477
- DOI: 10.1007/s10162-018-0662-z
Keratinocyte Growth Factor (KGF) Modulates Epidermal Progenitor Cell Kinetics through Activation of p63 in Middle Ear Cholesteatoma
Abstract
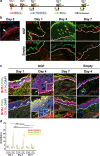
The basal stem/progenitor cell maintains homeostasis of the epidermis. Progressive disturbance of this homeostasis has been implicated as a possible cause in the pathogenesis of epithelial disease, such as middle ear cholesteatoma. In many cases of stem/progenitor cell regulation, the importance of extracellular signals provided by the surrounding cells is well-recognized. Keratinocyte growth factor (KGF) is a mesenchymal-cell-derived paracrine growth factor that specifically participates in skin homeostasis; however, the overexpression of KGF induces middle ear cholesteatoma. In this study, two kinds of thymidine analogs were transferred at different time points and we investigated the effects of overexpressed KGF on the cell kinetics of stem/progenitor cells in vivo. As a result, BrdU(+)EdU(+) cells (stem/progenitor cells) were detected in the thickened epithelium of KGF-transfected specimens. The use of a high-resolution microscope enabled us to analyze the phosphorylated level of p63 in individual nuclei, and the results clearly demonstrated that BrdU(+)EdU(+) cells are regarded as progenitor cells. In the overexpression of KGF, the stimulation of progenitor cell proliferation was inhibited by SU5402, an inhibitor for tyrosine kinase of KGFR. These findings indicate that KGF overexpression may increase stem/progenitor cell proliferation and block terminal differentiation, resulting in epithelial hyperplasia, which is typical in middle ear cholesteatoma.
Keywords: cell tracing; high-resolution microscopy; keratinocyte growth factor (KGF); middle ear cholesteatoma; p63 phosphorylation; progenitor cell.
Conflict of interest statement
Conflict of Interest
This study is supported by a Grant-in-Aid for Scientific Research from the Japanese Society for the Promotor of Science (JSPS) (no. 25462647 and JP16K11186 to T. Yamamoto-Fukuda, no. JP26462608 to N. Akiyama).
Figures





Similar articles
-
Keratinocyte growth factor (KGF) induces stem/progenitor cell growth in middle ear mucosa.Int J Pediatr Otorhinolaryngol. 2020 Jan;128:109699. doi: 10.1016/j.ijporl.2019.109699. Epub 2019 Oct 4. Int J Pediatr Otorhinolaryngol. 2020. PMID: 31614241
-
Keratinocyte growth factor signaling promotes stem/progenitor cell proliferation under p63 expression during middle ear cholesteatoma formation.Curr Opin Otolaryngol Head Neck Surg. 2020 Oct;28(5):291-295. doi: 10.1097/MOO.0000000000000655. Curr Opin Otolaryngol Head Neck Surg. 2020. PMID: 32796271 Review.
-
Possible involvement of keratinocyte growth factor and its receptor in enhanced epithelial-cell proliferation and acquired recurrence of middle-ear cholesteatoma.Lab Invest. 2003 Jan;83(1):123-36. doi: 10.1097/01.lab.0000050763.64145.cb. Lab Invest. 2003. PMID: 12533693
-
[Keratinocyte growth factor and its receptor in middle-ear cholesteatoma].Otolaryngol Pol. 2013 Mar-Apr;67(2):67-71. doi: 10.1016/j.otpol.2012.11.002. Epub 2012 Nov 23. Otolaryngol Pol. 2013. PMID: 23452652 Polish.
-
Keratinocyte growth factor expression and activity in cancer: implications for use in patients with solid tumors.J Natl Cancer Inst. 2006 Jun 21;98(12):812-24. doi: 10.1093/jnci/djj228. J Natl Cancer Inst. 2006. PMID: 16788155 Review.
Cited by
-
Regulation of DNA methylation levels in the process of oral mucosal regeneration in a rat oral ulcer model.Histol Histopathol. 2020 Mar;35(3):247-256. doi: 10.14670/HH-18-147. Epub 2019 Jul 9. Histol Histopathol. 2020. PMID: 31286466
-
The Role of Fgf Signaling on Epithelial Cell Differentiation in Mouse Vagina.In Vivo. 2019 Sep-Oct;33(5):1499-1505. doi: 10.21873/invivo.11630. In Vivo. 2019. PMID: 31471398 Free PMC article.
-
Analysis of the epidermal growth factor receptor/phosphoinositide-dependent protein kinase-1 axis in tumor of the external auditory canal in response to epidermal growth factor stimulation.Laryngoscope Investig Otolaryngol. 2022 Mar 30;7(3):730-739. doi: 10.1002/lio2.785. eCollection 2022 Jun. Laryngoscope Investig Otolaryngol. 2022. PMID: 35734041 Free PMC article.
-
Analysis of mRNA m6A modification and mRNA expression profiles in middle ear cholesteatoma.Front Genet. 2023 Aug 7;14:1188048. doi: 10.3389/fgene.2023.1188048. eCollection 2023. Front Genet. 2023. PMID: 37609036 Free PMC article.
-
The Effect of Zbxz23ir-21 NANO(nanomaterials) Delivery Vector on Apoptosis and PTEN(phosphatase and tensin homolog deleted on chromosome ten)/PI3K(Intracellular phosphatidylinositol kinase)/AKT(related to the A and C kinase) in Children with CHOLESTEATOMA in Middle Ear.Bioengineered. 2021 Dec;12(1):8809-8821. doi: 10.1080/21655979.2021.1984718. Bioengineered. 2021. PMID: 34696703 Free PMC article.
References
-
- Bao S, Wang Y, Sweeney P, Chaudhuri A, Doseff AI, Marsh CB, Knoell DL (2005) Keratinocyte growth factor induces Akt kinase activity and inhibits Fas-mediated apoptosis in A549 lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 288(1):L36–42. doi:0.1152/ajplung.00309.2003 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

