Diverse roles of mitochondria in ischemic stroke
- PMID: 29549824
- PMCID: PMC5854930
- DOI: 10.1016/j.redox.2018.03.002
Diverse roles of mitochondria in ischemic stroke
Abstract
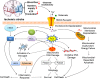
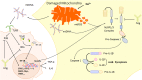
Stroke is the leading cause of adult disability and mortality in most developing and developed countries. The current best practices for patients with acute ischemic stroke include intravenous tissue plasminogen activator and endovascular thrombectomy for large-vessel occlusion to improve clinical outcomes. However, only a limited portion of patients receive thrombolytic therapy or endovascular treatment because the therapeutic time window after ischemic stroke is narrow. To address the current shortage of stroke management approaches, it is critical to identify new potential therapeutic targets. The mitochondrion is an often overlooked target for the clinical treatment of stroke. Early studies of mitochondria focused on their bioenergetic role; however, these organelles are now known to be important in a wide range of cellular functions and signaling events. This review aims to summarize the current knowledge on the mitochondrial molecular mechanisms underlying cerebral ischemia and involved in reactive oxygen species generation and scavenging, electron transport chain dysfunction, apoptosis, mitochondrial dynamics and biogenesis, and inflammation. A better understanding of the roles of mitochondria in ischemia-related neuronal death and protection may provide a rationale for the development of innovative therapeutic regimens for ischemic stroke and other stroke syndromes.
Keywords: Apoptosis; Inflammation; Ischemic stroke; Mitochondria; Mitochondrial biogenesis; Mitochondrial dynamics; Mitophagy.
Copyright © 2018 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Hsieh F.I., Lien L.M., Chen S.T., Bai C.H., Sun M.C., Tseng H.P., Chen Y.W., Chen C.H., Jeng J.S., Tsai S.Y. Get with the guidelines-stroke performance indicators: surveillance of stroke care in the taiwan stroke registry: get with the guidelines-stroke in Taiwan. Circulation. 2010;122:1116–1123. - PubMed
-
- Schwamm L.H., Fonarow G.C., Reeves M.J., Pan W., Frankel M.R., Smith E.E., Ellrodt G., Cannon C.P., Liang L., Peterson E. Get with the guidelines-stroke is associated with sustained improvement in care for patients hospitalized with acute stroke or transient ischemic attack. Circulation. 2009;119:107–115. - PubMed
-
- National Institute of Neurological D., Stroke rt P.A.S.S.G. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333:1581–1587. - PubMed
-
- Jauch E.C., Saver J.L., Adams H.P., Jr, Bruno A., Connors J.J., Demaerschalk B.M., Khatri P., McMullan P.W., Jr, Qureshi A.I., Rosenfield K. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2013;44:870–947. - PubMed
-
- Reeves M.J., Arora S., Broderick J.P., Frankel M., Heinrich J.P., Hickenbottom S., Karp H., LaBresh K.A., Malarcher A., Mensah G. Acute stroke care in the us: results from 4 pilot prototypes of the paul coverdell national acute stroke registry. Stroke. 2005;36:1232–1240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

