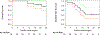Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors
- PMID: 29550207
- PMCID: PMC6357770
- DOI: 10.1016/j.athoracsur.2018.02.030
Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors
Abstract
Background: Surgeons are increasingly asked to operate on patients with residual disease after immunotherapy. The safety and utility of lung resection in this setting are unknown.
Methods: We retrospectively reviewed patients who underwent lung resection within 6 months of treatment with checkpoint blockade agents for metastatic or unresectable cancer. Survival was estimated from the first resection using the Kaplan-Meier approach.
Results: Database query identified 19 patients who underwent 22 resections for suspected residual disease with therapeutic intent after immunotherapy between 2012 and 2016. Lung cancer was the most common diagnosis (47%), followed by metastatic melanoma (37%). The most frequently used agents were nivolumab (32%), pembrolizumab (32%), and ipilimumab (16%). Patients received a mean of 21 doses (range, 1 to 70 doses). The final dose was administered at an average of 75 days (range, 7 to 183 days) before the operation. Anatomic resection (lobectomy or greater) was performed in 11 patients (50%). Four lobectomies were attempted minimally invasively, and one required conversion to thoracotomy. Of the resected patients, 68% had viable tumor remaining. R0 resection was achieved in 95%. Mean operative time for lobectomy was 227 minutes (range, 150 to 394 minutes). Complications occurred in 32% of patients; all but 1 were minor (grade 1/2). The 2-year overall and disease-free survival were 77% and 42%, respectively.
Conclusions: In patients with previously metastatic or unresectable cancer, lung resection for suspected residual disease after immunotherapy is feasible, with high rates of R0 resection. Operations can be technically challenging, but significant morbidity appears to be rare. Outcomes are encouraging, with reasonable survivals during short-interval follow-up.
Copyright © 2018 The Society of Thoracic Surgeons. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Comment in
-
Neoadjuvant PD-1 blockade in non-small cell lung cancer: what else do we need to do?J Thorac Dis. 2018 Sep;10(Suppl 26):S3162-S3165. doi: 10.21037/jtd.2018.07.84. J Thorac Dis. 2018. PMID: 30430027 Free PMC article. No abstract available.
-
Lung resection and immunotherapy: two allied for a new hope in lung cancer cure.J Thorac Dis. 2018 Nov;10(Suppl 33):S4168-S4169. doi: 10.21037/jtd.2018.10.93. J Thorac Dis. 2018. PMID: 30631584 Free PMC article. No abstract available.
-
Changing paradigms of non-small cell lung cancer treatment.J Thorac Dis. 2018 Nov;10(Suppl 33):S4170-S4172. doi: 10.21037/jtd.2018.11.04. J Thorac Dis. 2018. PMID: 30631585 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical