Estrogen receptor beta modulates permeability transition in brain mitochondria
- PMID: 29550215
- PMCID: PMC5912174
- DOI: 10.1016/j.bbabio.2018.03.006
Estrogen receptor beta modulates permeability transition in brain mitochondria
Abstract
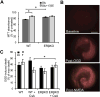
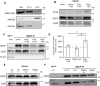
Recent evidence highlights a role for sex and hormonal status in regulating cellular responses to ischemic brain injury and neurodegeneration. A key pathological event in ischemic brain injury is the opening of a mitochondrial permeability transition pore (MPT) induced by excitotoxic calcium levels, which can trigger irreversible damage to mitochondria accompanied by the release of pro-apoptotic factors. However, sex differences in brain MPT modulation have not yet been explored. Here, we show that mitochondria isolated from female mouse forebrain have a lower calcium threshold for MPT than male mitochondria, and that this sex difference depends on the MPT regulator cyclophilin D (CypD). We also demonstrate that an estrogen receptor beta (ERβ) antagonist inhibits MPT and knockout of ERβ decreases the sensitivity of mitochondria to the CypD inhibitor, cyclosporine A. These results suggest a functional relationship between ERβ and CypD in modulating brain MPT. Moreover, co-immunoprecipitation studies identify several ERβ binding partners in mitochondria. Among these, we investigate the mitochondrial ATPase as a putative site of MPT regulation by ERβ. We find that previously described interaction between the oligomycin sensitivity-conferring subunit of ATPase (OSCP) and CypD is decreased by ERβ knockout, suggesting that ERβ modulates MPT by regulating CypD interaction with OSCP. Functionally, in primary neurons and hippocampal slice cultures, modulation of ERβ has protective effects against glutamate toxicity and oxygen glucose deprivation, respectively. Taken together, these results reveal a novel pathway of brain MPT regulation by ERβ that could contribute to sex differences in ischemic brain injury and neurodegeneration.
Keywords: Calcium; Cyclophilin D; Estrogen receptor; Mitochondria; Permeability transition.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures
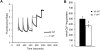




References
-
- M. Writing Group. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J, C. American Heart Association Statistics, S. Stroke Statistics Heart disease and stroke statistics–2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. - PubMed
-
- Girijala RL, Sohrabji F, Bush RL. Sex differences in stroke: Review of current knowledge and evidence. Vasc Med. 2017;22:135–145. - PubMed
-
- Racay P, Tatarkova Z, Chomova M, Hatok J, Kaplan P, Dobrota D. Mitochondrial calcium transport and mitochondrial dysfunction after global brain ischemia in rat hippocampus. Neurochem Res. 2009;34:1469–1478. - PubMed
-
- Starkov AA, Chinopoulos C, Fiskum G. Mitochondrial calcium and oxidative stress as mediators of ischemic brain injury. Cell Calcium. 2004;36:257–264. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

