Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update
- PMID: 29550566
- PMCID: PMC6057479
- DOI: 10.1016/j.ejca.2018.02.012
Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update
Erratum in
-
Corrigendum to 'Cabozantinib versus sunitinib as initial therapy for metastatic renal cell carcinoma of intermediate or poor risk (Alliance A031203 CABOSUN randomised trial): Progression-free survival by independent review and overall survival update' [Eur J Cancer 94 (May 2018) 115-125].Eur J Cancer. 2018 Nov;103:287. doi: 10.1016/j.ejca.2018.09.022. Epub 2018 Sep 27. Eur J Cancer. 2018. PMID: 30270112 Free PMC article. No abstract available.
Abstract
Background: The randomised phase 2 CABOSUN trial comparing cabozantinib with sunitinib as initial therapy for advanced renal cell carcinoma (RCC) of intermediate or poor risk met the primary end-point of improving progression-free survival (PFS) as assessed by investigator. We report PFS by independent radiology review committee (IRC) assessment, ORR per IRC and updated overall survival (OS).
Patients and methods: Previously untreated patients with advanced RCC of intermediate or poor risk by IMDC criteria were randomised 1:1 to cabozantinib 60 mg daily or sunitinib 50 mg daily (4 weeks on/2 weeks off). Stratification was by risk group and presence of bone metastases.
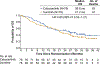
Results: A total of 157 patients were randomised 1:1 to cabozantinib (n = 79) or sunitinib (n = 78). Median PFS per IRC was 8.6 months (95% confidence interval [CI] 6.8-14.0) versus 5.3 months (95% CI 3.0-8.2) for cabozantinib versus sunitinib (hazard ratio [HR] 0.48 [95% CI 0.31-0.74]; two-sided p = 0.0008), and ORR per IRC was 20% (95% CI 12.0-30.8) versus 9% (95% CI 3.7-17.6), respectively. Subgroup analyses of PFS by stratification factors and MET tumour expression were consistent with results for the overall population. With a median follow-up of 34.5 months, median OS was 26.6 months (95% CI 14.6-not estimable) with cabozantinib and 21.2 months (95% CI 16.3-27.4) with sunitinib (HR 0.80 [95% CI 0.53-1.21]. The incidence of grade 3 or 4 adverse events was 68% for cabozantinib and 65% for sunitinib.
Conclusions: In this phase 2 trial, cabozantinib treatment significantly prolonged PFS per IRC compared with sunitinib as initial systemic therapy for advanced RCC of poor or intermediate risk.
Trial registration number: NCT01835158.
Keywords: Advanced renal cell carcinoma; Cabozantinib; First-line; IMDC risk groups; Sunitinib.
Copyright © 2018 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Choueiri TK, Motzer RJ. Systemic therapy for metastatic renalcell carcinoma. N Engl J Med 2017;376(4):354—66. - PubMed
-
- Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369(8):722—31. - PubMed
-
- Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356(2):115—24. - PubMed
-
- Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 2010;28(6):1061—8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

