Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012
- PMID: 29550834
- PMCID: PMC6170392
- DOI: 10.1038/s41375-018-0071-7
Successes and challenges in the treatment of pediatric acute myeloid leukemia: a retrospective analysis of the AML-BFM trials from 1987 to 2012
Abstract
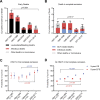
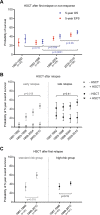
Overall survival (OS) of pediatric patients with acute myeloid leukemia (AML) increased in recent decades. However, it remained unknown whether advances in first-line treatment, supportive care, or second-line therapy mainly contributed to this improvement. Here, we retrospectively analyzed outcome and clinical data of 1940 pediatric AML patients (younger than 18 years of age), enrolled in the population-based AML-BFM trials between 1987 and 2012. While 5-year probability of OS (pOS) increased from 49 ± 3% (1987-1992) to 76 ± 4% (2010-2012; p < 0.0001), probability of event-free survival only improved from 41 ± 3% (1987-1992) to 50 ± 2% (1993-1998; p = 0.02) after introduction of high-dose cytarabine/mitoxantrone, but remained stable since then. Non-response and relapse rates stayed constant despite intensified first-line therapy (p = 0.08 and p = 0.17). Reduced fatal bleedings and leukostasis translated into fewer early deaths (8.1%vs. 2.2%; p = 0.001). Strikingly, pOS after non-response (13 ± 5% (1987-1992) vs. 43 ± 7% (2005-2010); p < 0.0001) or relapse (19 ± 4% vs. 45 ± 4%; p < 0.0001) improved. After 1999, more relapsed or refractory patients underwent hematopoietic stem cell transplantation (HSCT) with increased pOS after HSCT (29 ± 5% (1993-1998) vs. 50 ± 4% (2005-2010); p < 0.0001). Since efficacy of salvage therapy mainly contributed to better outcome in pediatric AML, our analysis indicates that a better allocation of patients, who cannot be cured with conventional chemotherapy, to an early "salvage-like" therapy is necessary.
Conflict of interest statement
J.-P.B. has consulting or advisory roles for Amgen and Roche. T.K. has consulting or advisory roles for Loxo and Novartis. T.L. receives honoraria from Gilead, Merk Sharp & Dome, Astella, Basilea, and has consulting or advisory roles and participates in speakers’ bureaus, including travel accommodation and other expenses from Gilead, Merk Sharp & Dome, Astella. T.L. receives research funding from Gilead. D.R. has consulting or advisory roles for Roche, Celgene, Hexal, Pfizer, Novartis, Boehringer and receives research funding from Celgene. D.R. received travel, accommodation or other expenses from Jazz Pharmaceuticals and Griffols. The remaining authors declare that they have no conflict of interest.
Figures



References
-
- Creutzig U, Zimmermann M, Bourquin JP, Dworzak MN, Fleischhack G, Graf N, et al. Randomized trial comparing liposomal daunorubicin with idarubicin as induction for pediatric acute myeloid leukemia: results from Study AML-BFM 2004. Blood. 2013;122:37–43. doi: 10.1182/blood-2013-02-484097. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

