Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells
- PMID: 29550884
- PMCID: PMC5937883
- DOI: 10.1007/s00415-018-8830-y
Cladribine treatment of multiple sclerosis is associated with depletion of memory B cells
Abstract
Background: The mechanism of action of oral cladribine, recently licensed for relapsing multiple sclerosis, is unknown.
Objective: To determine whether cladribine depletes memory B cells consistent with our recent hypothesis that effective, disease-modifying treatments act by physical/functional depletion of memory B cells.
Methods: A cross-sectional study examined 40 people with multiple sclerosis at the end of the first cycle of alemtuzumab or injectable cladribine. The relative proportions and absolute numbers of peripheral blood B lymphocyte subsets were measured using flow cytometry. Cell-subtype expression of genes involved in cladribine metabolism was examined from data in public repositories.
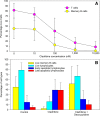
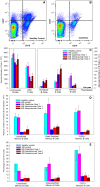
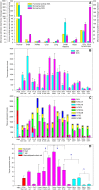
Results: Cladribine markedly depleted class-switched and unswitched memory B cells to levels comparable with alemtuzumab, but without the associated initial lymphopenia. CD3+ T cell depletion was modest. The mRNA expression of metabolism genes varied between lymphocyte subsets. A high ratio of deoxycytidine kinase to group I cytosolic 5' nucleotidase expression was present in B cells and was particularly high in mature, memory and notably germinal centre B cells, but not plasma cells.
Conclusions: Selective B cell cytotoxicity coupled with slow repopulation kinetics results in long-term, memory B cell depletion by cladribine. These may offer a new target, possibly with potential biomarker activity, for future drug development.
Keywords: B cell; Cladribine; Deoxycytidine kinase; Disease-modifying treatment; Memory B cells; Multiple sclerosis.
Conflict of interest statement
Conflicts of interest
GG has received fees for participation in advisory board and speaker fees for Merck. KS has received honoraria and meeting support from Merck. However, Merck was not involved in this study design or its implementation. Other disclosures are considered not relevant but: BC has nothing to declare, BMJ has nothing to declare; DB is a shareholder and consultant to Canbex therapeutics and has received research support from Sanofi-Genzyme; ND has nothing to declare; ZM has nothing to declare; GG has received fees for participation in advisory board from AbbVie Biotherapeutics, Biogen, Canbex, Ironwood, Novartis, Merck, Roche, Sanofi-Genzyme, Synthon, Teva and Vertex; speaker fees from AbbVie, Biogen, Bayer HealthCare, Genzyme, Sanofi-Aventis and Teva. Research support from Biogen, Genzyme, Ironwood, Merck, Merck Serono, Novartis and Takeda. KS has been a PI of trials sponsored by Novartis, Roche and Teva and involved in trials sponsored by Biogen, Sanofi-Genzyme, BIAL, Cytokinetics, and Canbex and has received honoraria and meeting support from Biogen, Novartis, and Teva.
Ethical standard statement
The study was approved by the Health and Social Care Research Ethics Committee B and the Health Research Authority, UK. People were recruited following informed consent. Purchased blood samples were collected with informed consent and did not require additional ethical review.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

